Electron Dot Structures 1 1. Use electron dot structures to show the transfer of electrons in the formation of the ionic compound, gallium fluoride. Ga :#: Gao 2. Write electron dot structures for the following species (assume all exhibit covalent bonding): CC4 SiO2 2- SO2 1ge 271 Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision)
Electron Dot Structures 1 1. Use electron dot structures to show the transfer of electrons in the formation of the ionic compound, gallium fluoride. Ga :#: Gao 2. Write electron dot structures for the following species (assume all exhibit covalent bonding): CC4 SiO2 2- SO2 1ge 271 Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision)
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 110E: Predict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a....
Related questions
Question
All of it
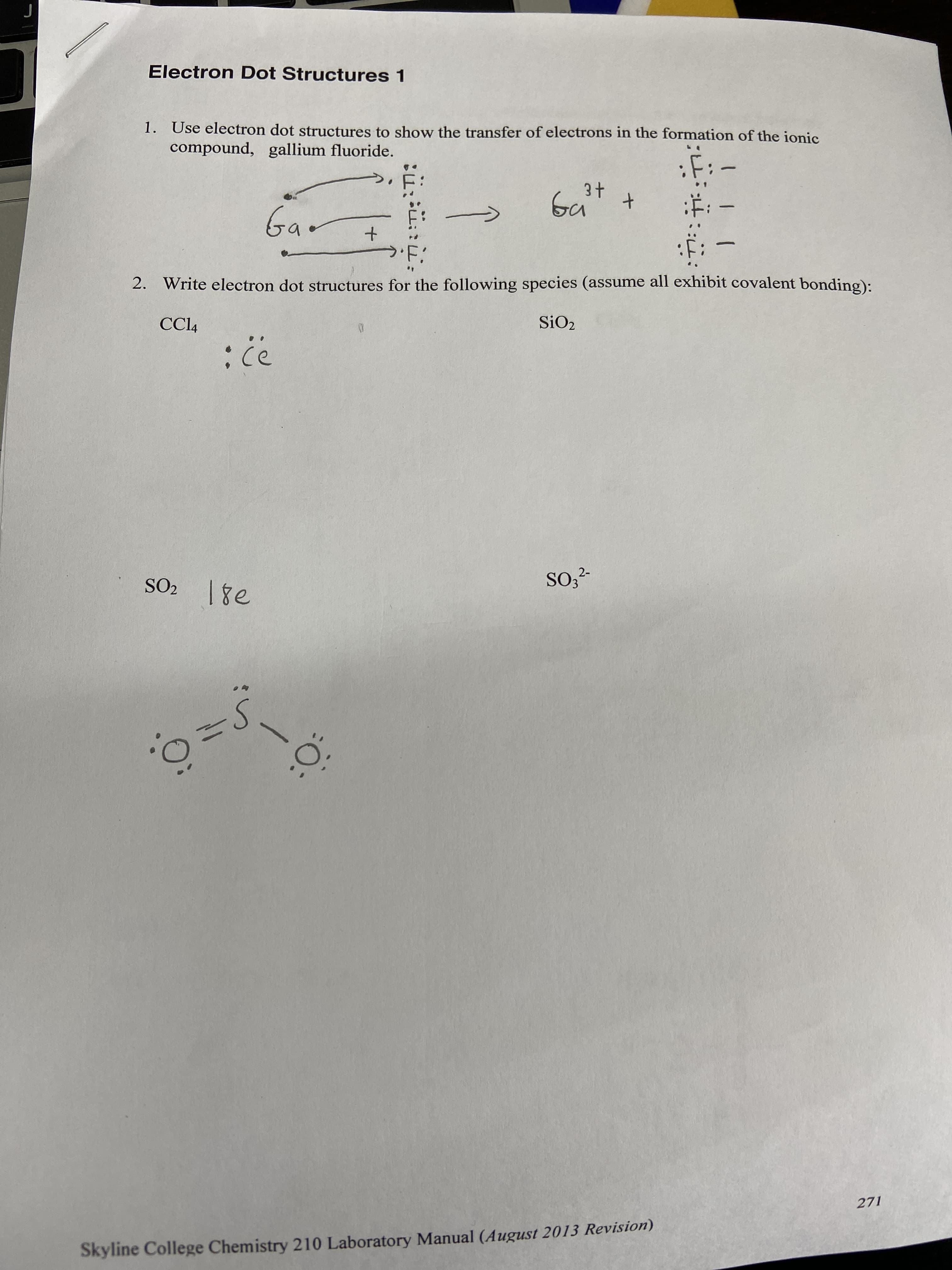
Transcribed Image Text:Electron Dot Structures 1
1. Use electron dot structures to show the transfer of electrons in the formation of the ionic
compound, gallium fluoride.
Ga
:#:
Gao
2. Write electron dot structures for the following species (assume all exhibit covalent bonding):
CC4
SiO2
2-
SO2 1ge
271
Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning