Emission of Light from Hydrogen and Metal Atoms A (meter) yim) V.138 3 1.11 1 0.0015 20S475 0.21 5 O.04 23 03100 0.02 10,12 6 2518022 4. Calculate the change in energy for each transition, ", 3,4, 5,6 and n, 2. Show your work and give the results in units of joules. 71 E (0.076X30X#) paaryle ")( hc ΔΕ. 1 = hcR Equation 3 п; H 71 3-2: AE = 3.02x10-19 T)(LMXLUA 1)(ADX00 E1(e ANaz9 4 2: AE = 4.09x10-1 J 4.511017 J 52: AE= 4.7 XIu J 6 2: AE= .85 x10-M Derive Fquation 3 from Equation 1: 4
Emission of Light from Hydrogen and Metal Atoms A (meter) yim) V.138 3 1.11 1 0.0015 20S475 0.21 5 O.04 23 03100 0.02 10,12 6 2518022 4. Calculate the change in energy for each transition, ", 3,4, 5,6 and n, 2. Show your work and give the results in units of joules. 71 E (0.076X30X#) paaryle ")( hc ΔΕ. 1 = hcR Equation 3 п; H 71 3-2: AE = 3.02x10-19 T)(LMXLUA 1)(ADX00 E1(e ANaz9 4 2: AE = 4.09x10-1 J 4.511017 J 52: AE= 4.7 XIu J 6 2: AE= .85 x10-M Derive Fquation 3 from Equation 1: 4
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter12: Atoms And Molecules
Section: Chapter Questions
Problem 12.46E: Explain why assuming an effective nuclear charge, as used for our treatment of the helium atom in...
Related questions
Question
100%
how do I fill out the box?
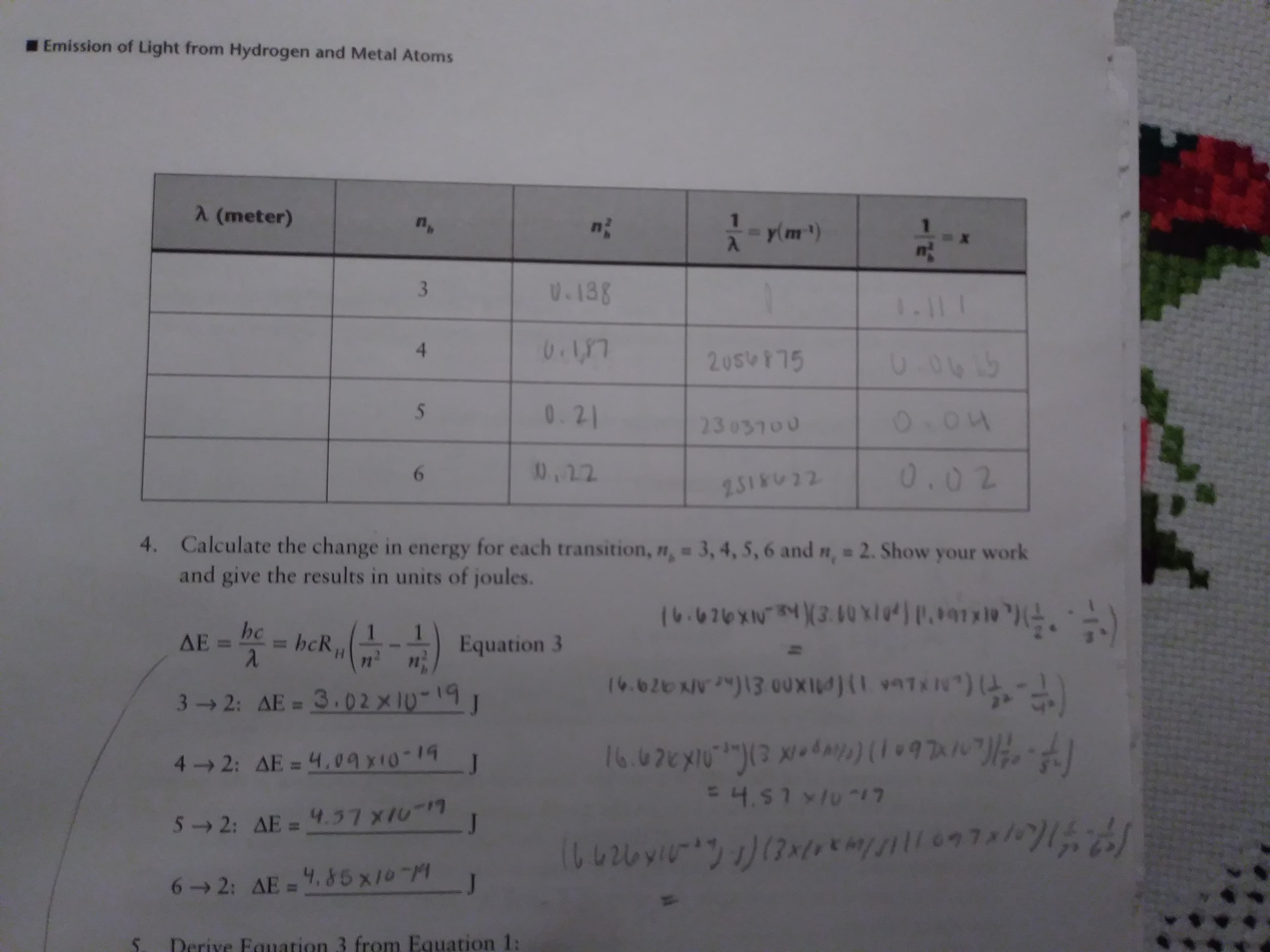
Transcribed Image Text:Emission of Light from Hydrogen and Metal Atoms
A (meter)
yim)
V.138
3
1.11 1
0.0015
20S475
0.21
5
O.04
23 03100
0.02
10,12
6
2518022
4. Calculate the change in energy for each transition, ", 3,4, 5,6 and n, 2. Show your work
and give the results in units of joules.
71 E
(0.076X30X#) paaryle ")(
hc
ΔΕ.
1
= hcR
Equation 3
п;
H
71
3-2: AE = 3.02x10-19
T)(LMXLUA 1)(ADX00 E1(e ANaz9
4 2: AE = 4.09x10-1
J
4.511017
J
52: AE= 4.7 XIu
J
6 2: AE= .85 x10-M
Derive Fquation 3 from Equation 1:
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning