empirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Given pair of elements,
Q: Determine the number of Ca2+ and Br¯ ions required to form a neutral ionic compound. Cations Anions…
A:
Q: Name ionic compound. BaS
A: Given: BaS
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic bond is formed by the transfer of electron from an electropositive atom to the electronegative…
Q: rite the empirical formula for at least four ionic compounds that could be formed from the following…
A: The ions given are Pb4+, CO32-, Fe3+ and SO42-.
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A:
Q: Decide wieuner each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compounds are the neutral compounds in which the positively charged ions (cations) and…
Q: Wrether each pair of elements in the table below will form an ionic compound. If they will, write…
A: Ionic compounds are formed by transfer of electrons between metal and non metal
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they w…
A: Ionic compounds are formed by ions. The metal atom loses electrons and forms cations and the…
Q: Write the formula for Silver Sulfide (enter all subscripts as numbers, for example Al2O3 would be…
A: Formula of a compound is written by cation and anion in which they transfer charge by each other and…
Q: empirical formula of ionic compound Forms ionic element #1 element #2 compound? bromine lithium |yes…
A: Ionic compounds are the neutral compounds in which the positively charged ions (cations) and…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compound are formed by the combination of metal ions called cations and nonmetal ions called…
Q: Write the formulas for the ionic compounds formed between these ions. Make sure you account for the…
A: Iodide ion and magnesium ion The charge on the cation (Mg2+) becomes the subscript on the…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: An ionic compound is form by interaction of metal and non-metal a covalent compound is formed by…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: The ionic compound is formed between highly electropositive and highly electronegative elements.…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compound is formed when electron is transferred from anion to cation. While writing the…
Q: Some ionic compounds cation anion empirical formula name of compound Cu CN 3+ Co PO 2+ Fe co;
A: 1) CuCN - Copper(I) Cyanide 2) CoPO4 - Cobalt(|||) Phosphate 3)FeCO3 - Ferrous Carbonate or Iron(II)…
Q: Draw the Lewis structure of ionic compounds. • Determine the formula of the ionic compound formed…
A: “Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compound: When a electropositive element combines with high electronegative element, forms…
Q: O MEASUREMENT AND MATTER Predicting and naming ionic compounds formed by two elements Mortor 0/5…
A: Here we have to determine whether ionic compound formed or not between the pair of given elements…
Q: 4) Write the empirical formula for the simplest binary ionic compound formed from each ion or…
A: ionic compound The ionic compound is formed by the bond between Cation and the anion . To form an…
Q: Binary Covalent Polyatomic or ionic or ion? acid name If ionic, charge on the cation and anion…
A:
Q: empirical formula of ionic compound Forms ionic element #1 element #2 compound? fluorine O no ?…
A: Since you have asked for multiple question, as per our company guidelines we are supposed to answer…
Q: Name of Acid Cation Anion Formula of Acid 1. chlorous acid 2. sulfuric acid 3. phosphoric acid 4.…
A: Solution : Acids are hydrogen-containing substances that are capable of donating a proton. They…
Q: deca. Table 3. Covalent compound naming Name Formula Phosphorous PCI5 pentachloride Dihydrogen H2O…
A:
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A:
Q: Formula of the compound formed Ions involved Name of the compound Mg +2 and F- Rb + and 0 -2 Pb +4…
A: A chemical formula of an ionic compound can be formed by criss-crossing the valencies of their ions.…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A:
Q: Write formulas for compound formed by the following ions: K+ and O2- Ni2+ and HSO4- NH4+ and C2O42-…
A: Ionic compounds An ionic compound is a chemical compound composed of ions held together by…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: When a metal combine with non-metal , then ionic bonding is formed . Metal takes electrons to…
Q: Write the empirical formula of at least four binary ionic compounds that could be formed fro 2+ 4+…
A:
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compounds are formed by the transfer of electrons. The cation donates the electrons and the…
Q: Decide wnether each pair of elements in the table below will form an ionic compound. If they will,…
A: Answer
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compounds are formed when transfer of electrons takes place and the bond formed by these…
Q: Part 2 Writing Formulas-(20 Points)-use the prefix table for naming covalent compounds and the table…
A:
Q: Nomenclature A. Look at each formula in the list below and identify each As Acid (A), Ionic (I), or…
A: The inorganic compounds are named in accordance to the chemical nomenclature. Compounds containing 2…
Q: empirical formula of ionic compound Forms ionic element #1 element #2 compound? ? barium iodine yes…
A: The table is filled as follows:
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: The elements given are,
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Since you have posted multiple questions, we are entitled to answer the first only. Please repost…
Q: Fill in the name and empirical formula of each ionic compound that could be formed from the lons in…
A: Empirical formula can be defined as "The formula which gives the correct proportions of the each…
Q: element pair will form a molecular compound element #1 element #2 molecular chemical name compound…
A:
Q: empirical formula of ionic compound Forms ionic element #1 element #2 compound? iodine O yes ?…
A: since you have asked for multiple question, as per our company guidelines we are supposed to solve…
Q: rite the empirical formula of at least four binary ionic compounds that could be formed from the…
A: The formula of ionic compounds is written by following electrical neutrality principle According to…
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compounds are formed only between a non metal and a metal element If the bond is made between…
Q: Name Ions Formula sodium sulfate Na+1 sO4-2 So4-2 lithium phosphate aluminum acetate tin(II) nitrate…
A: Chemical formula for an ionic compound can be formed by criss-crossing the valencies of ions.
Q: State the name of the ionic compound formed between Ca2+ cation and the polyatomic anion SO42-.…
A: A question based on introduction to Chemistry that is to be accomplished.
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Cesium is a cation while bromine is an anion hence, they will form an ionic compound. Sodium is a…
Q: Naming ionić čompounds with common polyatomic ions Fill in the name and empirical formula of each…
A: The empirical formula and name of the compound has to be given.
Q: empirical Forms ionic element #1 element #2 formula of ionic name of ionic compound compound?…
A: Ionic compound form between the element 1 & 2 or not = ? if Yes, then formula of the ionic…
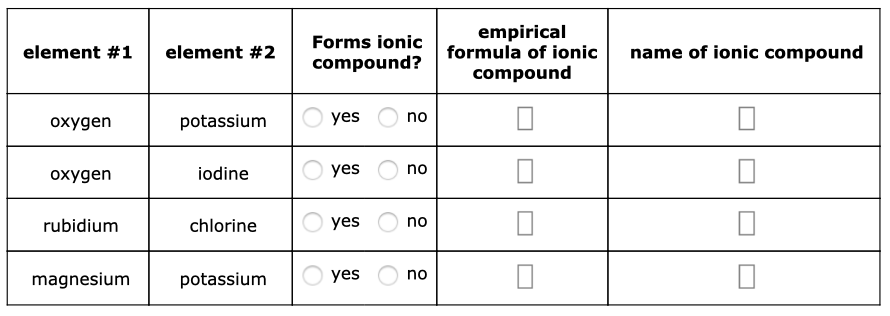
Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

- Mixing SbCl3 and GaCl3 in a 1;1 molar ratio (usingliquid sulfur dioxide as a solvent) gives a solid ioniccompound of empirical formula GaSbCl6. A controversyarises over whether this compound is ( ) SbCl2 + ( ) GaCl 4 - or( ) GaCl+ 2 ( ) SbCl 4 -(a) Predict the molecular structures of the two anions.(b) It is learned that the cation in the compound has abent structure. Based on this fact, which formulationis more likely to be correct?Findthemass%ofH2O2 inasolutionmadefrom36gofH2O2 and84gofH2O: A) 84% B) 36% C) 43% D) 30.% E) 70.%The attractive force between two ions in Na20, Fc is 8.64x10-9N. What is the bond length a0 (in nm) given that valence of the charged Na+ is Z1=+1, for O-, is Z2 =-2, the charge of a single electron q is 0.16x10-18 C and the proportionality constant k0=9x109 V.m/C? Note that K=k0(Z1q)(Z2q) and that 1 V.C = 1 J.
- Explain the trend in the strengths of oxyacids (Pauling’s prediction)A major challenge in implementing the “hydrogen economy”is finding a safe, lightweight, and compact way of storinghydrogen for use as a fuel. The hydrides of light metalsare attractive for hydrogen storage because they can store ahigh weight percentage of hydrogen in a small volume. Forexample, NaAlH4 can release 5.6% of its mass as H2 upondecomposing to NaH(s), Al(s), and H2(g). NaAlH4 possessesboth covalent bonds, which hold polyatomic anionstogether, and ionic bonds. (a) Write a balanced equationfor the decomposition of NaAlH4. (b) Which element inNaAlH4 is the most electronegative? Which one is the leastelectronegative? (c) Based on electronegativity differences,predict the identity of the polyatomic anion. Draw a Lewisstructure for this ion. (d) What is the formal charge on hydrogenin the polyatomic ion?A major challenge in implementing the “hydrogen economy”is finding a safe, lightweight, and compact way of storinghydrogen for use as a fuel. The hydrides of light metalsare attractive for hydrogen storage because they can store ahigh weight percentage of hydrogen in a small volume. Forexample, NaAlH4 can release 5.6% of its mass as H2 upondecomposing to NaH1s2, Al1s2, and H21g2. NaAlH4 possessesboth covalent bonds, which hold polyatomic anionstogether, and ionic bonds. (a) Write a balanced equationfor the decomposition of NaAlH4. (b) Which element inNaAlH4 is the most electronegative? Which one is the leastelectronegative? (c) Based on electronegativity differences,predict the identity of the polyatomic anion. Draw a Lewisstructure for this ion. (d) What is the formal charge on hydrogenin the polyatomic ion?
- Give the name and the configuration of the following substance bellowAbout Fajans proposal, polarizability and strength of ionic/covelent bond Select one: 1-according to Fajans proposal as the ionic radius decreases the polarization power of an ion increases and vice versa hence the order of polarizing power of the alkaline metals according to the decline in their atomic radius Li+>Na+>K+ and declines as going down this IA group 2- according to Fajans proposal as the ionic charge increases the polarization power of an ion decreases and vice versa thus the order of polarizing power of metals according to their charges is as follows Al+3 > Mg+2 > Na+ 3- Charge (Q)/ radius (r) ratio determines the polarization effect or power of cations therefore, cations with large radius but small high have best polarization effect 4- according to Fajans proposal ionic charge, ionic radius and the valance shell structure are not related to the ionic bond strengthSuppose we introduce the following pointdefects.(a) Mg2+ ions substitute for yttrium ionsin Y2O3; (b) Fe3+ ions substitute for magnesiumions in MgO;(c) Li1+ ions substitute for magnesiumions in MgO; and(d) Fe2+ ions replace sodium ions inNaCl.What other changes in each structuremight be necessary to maintain a chargebalance? Explain.
- Calculate the lattice energy of AgBr(s) using the following thermodynamic data (all data is in kJ/mol). Ag(s) ΔHsublimation = 265 kJ/mol Ag(g) Ionization energy = 711 kJ/mol Br-Br(g) Bond energy = 173 kJ/mol Br(g) Electron affinity = -345 kJ/mol AgBr(s) ΔH°f = -120 kJ/mol ______________ kJ/molThionyl chloride (SOCl₂) is a sulfur oxohalide used indus-trially to dehydrate metal halide hydrates. (a) Write a balancedequation for its reaction with magnesium chloride hexahydrate,in which SO₂ and HCl form along with the metal halide. (b) Drawa Lewis structure of SOCl₂ with minimal formal charges.Iodine monochloride and elemental bromine have nearly the same molar mass and liquid density but very different boiling points. (a) What molecular property is primarily responsible forthis difference in boiling point? What atomic property gives rise to it? Explain. (b) Which substance has a higher boiling point?Why?

