EMPIRICAL GAS LAW T01/S03 An expandable weather balloon reached a height at which its volume was 74.5 L, the temperature was 306 K and the atmospheric pressure was 737 torr. If it ascended to a new height at which the atmospheric pressure was 593 torr and the volume was 5.61 x 104 mL, calculate the temperature (K). Unit conversion K = C + 273 х 10 1 atm = 760 torr EMPIRICAL GAS LAW T01/S02 An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and 37 °C ascended to a height at which the temperature was 18 (°C) and the volume was 10.5 L. Calculate the atmospheric pressure (torr). Unit conversion K = C + 273 х 10 1 atm = 760 torr
EMPIRICAL GAS LAW T01/S03 An expandable weather balloon reached a height at which its volume was 74.5 L, the temperature was 306 K and the atmospheric pressure was 737 torr. If it ascended to a new height at which the atmospheric pressure was 593 torr and the volume was 5.61 x 104 mL, calculate the temperature (K). Unit conversion K = C + 273 х 10 1 atm = 760 torr EMPIRICAL GAS LAW T01/S02 An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and 37 °C ascended to a height at which the temperature was 18 (°C) and the volume was 10.5 L. Calculate the atmospheric pressure (torr). Unit conversion K = C + 273 х 10 1 atm = 760 torr
Chapter5: Gases
Section: Chapter Questions
Problem 143CWP: A certain flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon...
Related questions
Question
Please help me with both parts, they are attached as 2 screenshots, make it clear what the answer to each part is, and double and triple check your answers previous tutors gave the wrong answers.
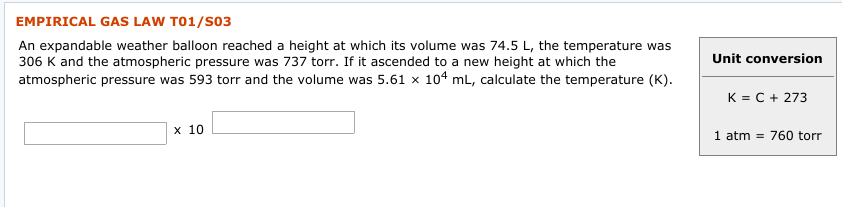
Transcribed Image Text:EMPIRICAL GAS LAW T01/S03
An expandable weather balloon reached a height at which its volume was 74.5 L, the temperature was
306 K and the atmospheric pressure was 737 torr. If it ascended to a new height at which the
atmospheric pressure was 593 torr and the volume was 5.61 x 104 mL, calculate the temperature (K).
Unit conversion
K = C + 273
х 10
1 atm = 760 torr

Transcribed Image Text:EMPIRICAL GAS LAW T01/S02
An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and 37 °C
ascended to a height at which the temperature was 18 (°C) and the volume was 10.5 L. Calculate the
atmospheric pressure (torr).
Unit conversion
K = C + 273
х 10
1 atm = 760 torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning