EMPIRICAL GAS LAW TO1/S02 An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and was 18 (°C) and the volume was 10.5 L. Calculate the atmospheric pressure (torr). 37 °C ascended to a height at which the temperature Unit conversion K = C + 273 х 10 1 atm = 760 torr 1 used Question Attempts: 0 of SUBMIT ANSWER SAVE FOR LATER EMPIRICAL GAS LAW T01/S01 Unit conversion A sample of air in a cylinder with a moveable piston has a 2200 ml volume at a pressure of 3650 torr and a temperature of 67 °C. Calculate the pressure (atm) of the gas when the volume changes to 3900 mL and the temperature is 387 K. K = C + 273 atm = 760 torr 1 the tolerance is +/-2% Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
EMPIRICAL GAS LAW TO1/S02 An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and was 18 (°C) and the volume was 10.5 L. Calculate the atmospheric pressure (torr). 37 °C ascended to a height at which the temperature Unit conversion K = C + 273 х 10 1 atm = 760 torr 1 used Question Attempts: 0 of SUBMIT ANSWER SAVE FOR LATER EMPIRICAL GAS LAW T01/S01 Unit conversion A sample of air in a cylinder with a moveable piston has a 2200 ml volume at a pressure of 3650 torr and a temperature of 67 °C. Calculate the pressure (atm) of the gas when the volume changes to 3900 mL and the temperature is 387 K. K = C + 273 atm = 760 torr 1 the tolerance is +/-2% Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.22E: Earths atmosphere is approximately 80 N2 and 20 O2. If the total atmospheric pressure at sea level...
Related questions
Question
Please help me with both parts, they are attached as 2 screenshots, make it clear what the answer to each part is, and double and triple check your answers previous tutors gave the wrong answers.
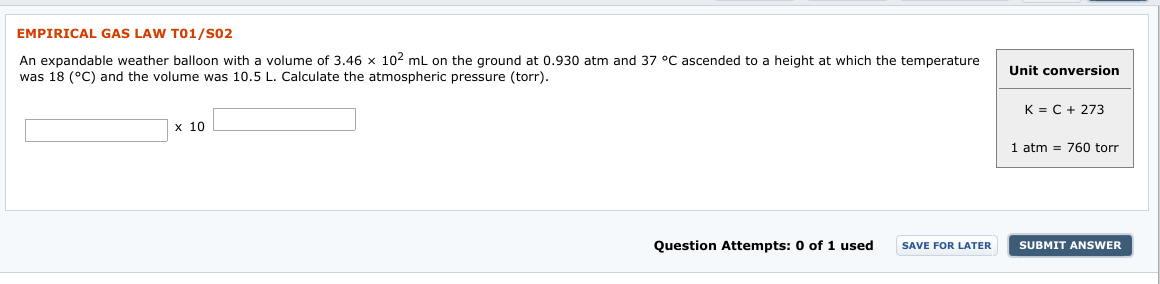
Transcribed Image Text:EMPIRICAL GAS LAW TO1/S02
An expandable weather balloon with a volume of 3.46 x 102 mL on the ground at 0.930 atm and
was 18 (°C) and the volume was 10.5 L. Calculate the atmospheric pressure (torr).
37 °C ascended to a height at which the temperature
Unit conversion
K = C + 273
х 10
1 atm = 760 torr
1 used
Question Attempts: 0 of
SUBMIT ANSWER
SAVE FOR LATER
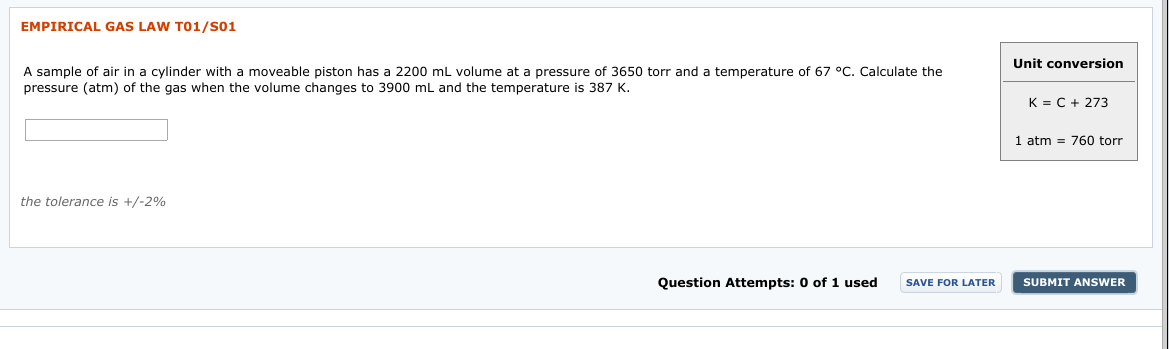
Transcribed Image Text:EMPIRICAL GAS LAW T01/S01
Unit conversion
A sample of air in a cylinder with a moveable piston has a 2200 ml volume at a pressure of 3650 torr and a temperature of 67 °C. Calculate the
pressure (atm) of the gas when the volume changes to 3900 mL and the temperature is 387 K.
K = C + 273
atm = 760 torr
1
the tolerance is +/-2%
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning