envellum.ecollege.com/course.html?courseld=15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001 Search... Ael. AOL Video- Serving the best vi.. nline Sh... TripAdvisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: To become familiar with the concept and calculations of specific heat. Part A Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g - °C). How much heat energy is required to raise the temperature of 0.360 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. > View Available Hint(s) Using SH for specific heat, the formula for calculating specific heat is HA Value Units heat heat = SH = massxAT P Pearson Terms of Use | Privacy Policy l Permissions l Contact Us 2019 Pearson Education Inc. All rights reserved. Copyright 8:50 PM 12/4/2019 Cip ins brt sc t9 144 fho fa delet & 7. %23 back 8. 4. Y. H. L. paus V. home ctrl alt Σ F. %24 %23 n/course.html?courseld315432274&HeplD=2b3e48e6520860bfd5591538a4a5a27b#10001 Search. deo - Serving the best vi.. ivisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: Part B To become familiar with the concept and calculations of specific heat. If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 °C, what will the final temperature be? The specific heat of copper is 0.0920 cal/(g- °C). Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. • View Available Hint(s) HA Using SH for specific heat, the formula for calculating specific heat is Value Tinal Units heat SH= massxAT Submit P Pearson Copyright © 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy Contact Us Permissions hp a 8:50 PM 12/4/2019 Cop fs f6 fg fg ho f11 ins prt sc delete 7. 8. backspace, R. T. Y. P. H. enter pause V. N. M. home %24
envellum.ecollege.com/course.html?courseld=15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001 Search... Ael. AOL Video- Serving the best vi.. nline Sh... TripAdvisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: To become familiar with the concept and calculations of specific heat. Part A Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g - °C). How much heat energy is required to raise the temperature of 0.360 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. > View Available Hint(s) Using SH for specific heat, the formula for calculating specific heat is HA Value Units heat heat = SH = massxAT P Pearson Terms of Use | Privacy Policy l Permissions l Contact Us 2019 Pearson Education Inc. All rights reserved. Copyright 8:50 PM 12/4/2019 Cip ins brt sc t9 144 fho fa delet & 7. %23 back 8. 4. Y. H. L. paus V. home ctrl alt Σ F. %24 %23 n/course.html?courseld315432274&HeplD=2b3e48e6520860bfd5591538a4a5a27b#10001 Search. deo - Serving the best vi.. ivisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: Part B To become familiar with the concept and calculations of specific heat. If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 °C, what will the final temperature be? The specific heat of copper is 0.0920 cal/(g- °C). Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. • View Available Hint(s) HA Using SH for specific heat, the formula for calculating specific heat is Value Tinal Units heat SH= massxAT Submit P Pearson Copyright © 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy Contact Us Permissions hp a 8:50 PM 12/4/2019 Cop fs f6 fg fg ho f11 ins prt sc delete 7. 8. backspace, R. T. Y. P. H. enter pause V. N. M. home %24
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 33PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
Transcribed Image Text:envellum.ecollege.com/course.html?courseld=15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001
Search...
Ael. AOL Video- Serving the best vi..
nline Sh... TripAdvisor
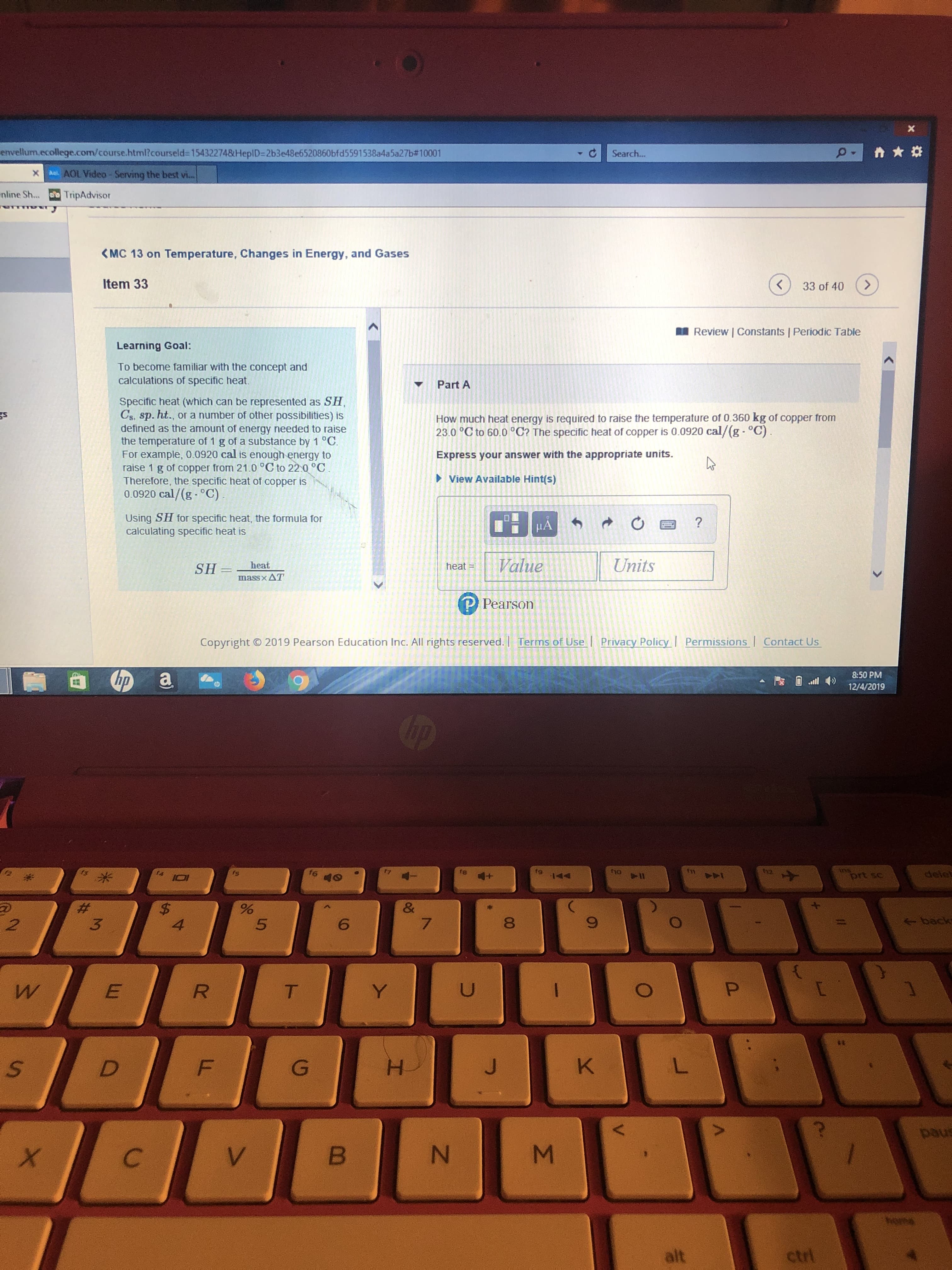
<MC 13 on Temperature, Changes in Energy, and Gases
Item 33
<>
33 of 40
I Review | Constants | Periodic Table
Learning Goal:
To become familiar with the concept and
calculations of specific heat.
Part A
Specific heat (which can be represented as SH,
Cs, sp. ht., or a number of other possibilities) is
defined as the amount of energy needed to raise
the temperature of 1 g of a substance by 1 °C.
For example, 0.0920 cal is enough energy to
raise 1 g of copper from 21.0 °C to 22.0 °C.
Therefore, the specific heat of copper is
0.0920 cal/(g - °C).
How much heat energy is required to raise the temperature of 0.360 kg of copper from
23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g- °C).
Express your answer with the appropriate units.
> View Available Hint(s)
Using SH for specific heat, the formula for
calculating specific heat is
HA
Value
Units
heat
heat =
SH =
massxAT
P Pearson
Terms of Use | Privacy Policy l Permissions l Contact Us
2019 Pearson Education Inc. All rights reserved.
Copyright
8:50 PM
12/4/2019
Cip
ins
brt sc
t9
144
fho
fa
delet
&
7.
%23
back
8.
4.
Y.
H.
L.
paus
V.
home
ctrl
alt
Σ
F.
%24
%23
Transcribed Image Text:n/course.html?courseld315432274&HeplD=2b3e48e6520860bfd5591538a4a5a27b#10001
Search.
deo - Serving the best vi..
ivisor
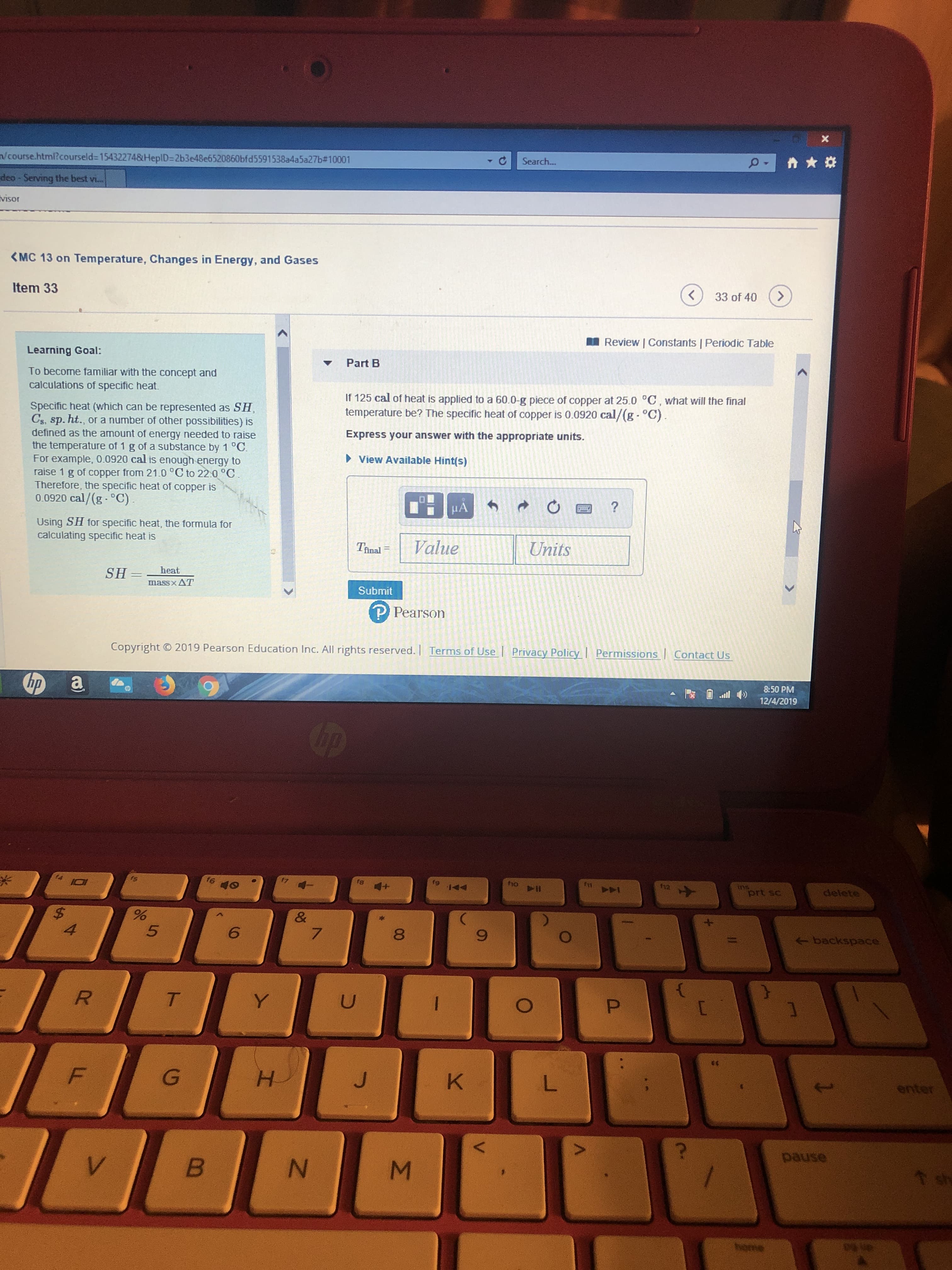
<MC 13 on Temperature, Changes in Energy, and Gases
Item 33
<>
33 of 40
I Review | Constants | Periodic Table
Learning Goal:
Part B
To become familiar with the concept and
calculations of specific heat.
If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 °C, what will the final
temperature be? The specific heat of copper is 0.0920 cal/(g- °C).
Specific heat (which can be represented as SH,
Cs, sp. ht., or a number of other possibilities) is
defined as the amount of energy needed to raise
the temperature of 1 g of a substance by 1 °C.
For example, 0.0920 cal is enough energy to
raise 1 g of copper from 21.0 °C to 22.0 °C.
Therefore, the specific heat of copper is
0.0920 cal/(g- °C).
Express your answer with the appropriate units.
• View Available Hint(s)
HA
Using SH for specific heat, the formula for
calculating specific heat is
Value
Tinal
Units
heat
SH=
massxAT
Submit
P Pearson
Copyright © 2019 Pearson Education Inc. All rights reserved.
Terms of Use
Privacy Policy
Contact Us
Permissions
hp a
8:50 PM
12/4/2019
Cop
fs
f6
fg
fg
ho
f11
ins
prt sc
delete
7.
8.
backspace,
R.
T.
Y.
P.
H.
enter
pause
V.
N.
M.
home
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,