estion 11 of 23 What is the number of formula units in a 1.88 mol sample of FeCl, ? formula units stion 8 of 23 <> O Ate Suppose you have two samples that are equal in weight, 28.3 g Ni and 28.3 g Fe, 02. Calculate the number of moles of each substance. Ni: 159.6 Fe, 03: 0.177 mo contact us help privacy policy terms of use about us careers
estion 11 of 23 What is the number of formula units in a 1.88 mol sample of FeCl, ? formula units stion 8 of 23 <> O Ate Suppose you have two samples that are equal in weight, 28.3 g Ni and 28.3 g Fe, 02. Calculate the number of moles of each substance. Ni: 159.6 Fe, 03: 0.177 mo contact us help privacy policy terms of use about us careers
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter7: Chemical Formula Relationships
Section: Chapter Questions
Problem 55E: Explain why C6H10 must be a molecular formula, whereas C7H10 could be a molecular formula, an...
Related questions
Question

Transcribed Image Text:estion 11 of 23
What is the number of formula units in a 1.88 mol sample of FeCl, ?
formula units
Transcribed Image Text:stion 8 of 23
<>
O Ate
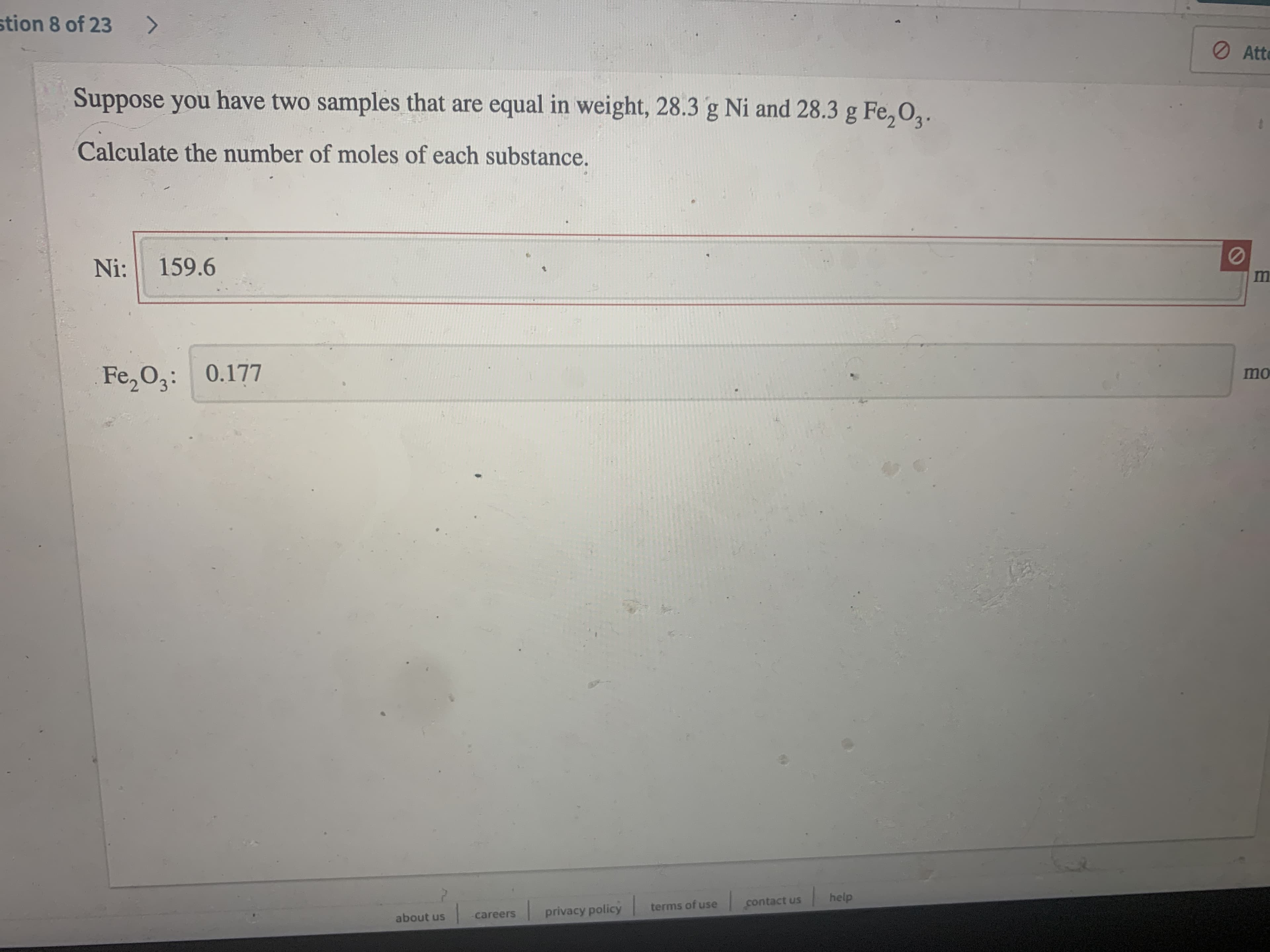
Suppose you have two samples that are equal in weight, 28.3 g Ni and 28.3 g Fe, 02.
Calculate the number of moles of each substance.
Ni:
159.6
Fe, 03: 0.177
mo
contact us
help
privacy policy
terms of use
about us
careers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning