Evib = @. + @.ye v+• +... %3D Molecule u (amu) k (Nm-') we(cm-1) Wxe (cm-1) wYe (cm¬1) 'H 1271 1.00 310 2309.60 39.36 -0.09 2H 1°F 1.82 960 2998.19 45.76 12C 160 6.86 1900 2169.82 13.29 0.01 14N !ªN 7.00 2290 2358.07 14.19 -0.01 14N 160+ 7.47 2490 2377.48 16.45 14N 160 7.47 1600 1904.41 14.19 0.02 14N 160- 7.47 830 1372 160 l60 8.00 1180 1580.36 12.07 0.05 19F 1ºF 9.50 440 891.2 35C1 5CI 17.48 320 560.50 2.90 0.02
Evib = @. + @.ye v+• +... %3D Molecule u (amu) k (Nm-') we(cm-1) Wxe (cm-1) wYe (cm¬1) 'H 1271 1.00 310 2309.60 39.36 -0.09 2H 1°F 1.82 960 2998.19 45.76 12C 160 6.86 1900 2169.82 13.29 0.01 14N !ªN 7.00 2290 2358.07 14.19 -0.01 14N 160+ 7.47 2490 2377.48 16.45 14N 160 7.47 1600 1904.41 14.19 0.02 14N 160- 7.47 830 1372 160 l60 8.00 1180 1580.36 12.07 0.05 19F 1ºF 9.50 440 891.2 35C1 5CI 17.48 320 560.50 2.90 0.02
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.23PAE
Related questions
Question
Calculate the vibrational energy of the v = 2 state of 1H127 I relative to the ground state, including the anharmonic corrections.
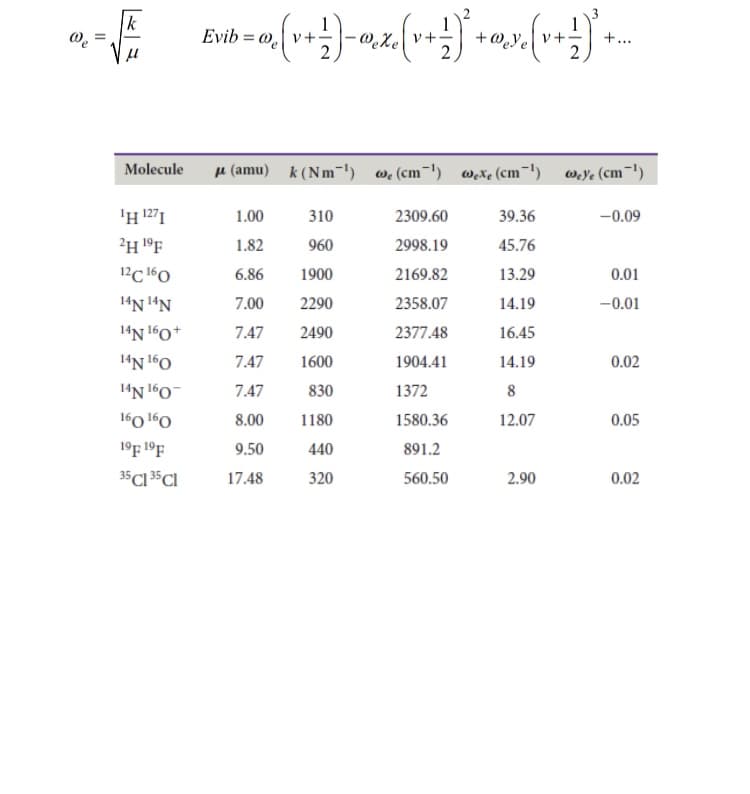
Transcribed Image Text:Evib = @.
+ @.ye v+•
+...
%3D
Molecule
u (amu) k (Nm-') we(cm-1) Wxe (cm-1) wYe (cm¬1)
'H 1271
1.00
310
2309.60
39.36
-0.09
2H 1°F
1.82
960
2998.19
45.76
12C 160
6.86
1900
2169.82
13.29
0.01
14N !ªN
7.00
2290
2358.07
14.19
-0.01
14N 160+
7.47
2490
2377.48
16.45
14N 160
7.47
1600
1904.41
14.19
0.02
14N 160-
7.47
830
1372
160 l60
8.00
1180
1580.36
12.07
0.05
19F 1ºF
9.50
440
891.2
35C1 5CI
17.48
320
560.50
2.90
0.02
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning