Q: Reaction: Pb(NO3)2 + Na2SO4 Molecular equation: Net ionic equation:
A: In molecular equation the chemical reaction is written in which compounds are present as molecules.…
Q: Many ionic compounds are water soluble, so, for example, we can make solutions of sodium iodide,…
A: Dissolution of solid ionic lead nitrate compound gives lead ions and nitrate ions. The overall…
Q: Hi, Please write reactions to balanced molecular equation, total ionic equation and net ionic…
A: The balanced molecular equation must contains all the molecules involve in the reaction in the form…
Q: Is the total ionic equation the same as the net ionic equation when Sr(OH)2(aq) and H2SO4(aq) react?…
A: Solution : A balanced ionic equation can be written in a way that the number and types of atoms…
Q: Ba(NO3)2(aq) + NaHCO3(aq) a. Full Molecular Equation: b. Full Ionic Equation: c. Net Ionic…
A: Concept introduction: Molecular form of equation represent the chemical equation in the molecular…
Q: a. Balanced formula equation: HNO3 (aq) + 1 Zn(OH), (s) Zn(NO,)2 + 2H,0 Complete ionic equation: Net…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: u(NO3)2(aq) + Na2 SO4(aq) a. Full Molecular Equation: b. Full Ionic Equation: c. Net Ionic…
A:
Q: Find the following the molecular equation, total ionic equation, and net ionic equation. Given…
A: The different equations are as follows:
Q: Question attached
A: In balanced equation the atoms of similar kind will be equal in number for reactant side and product…
Q: Write the net ionic equation for the following molecular equation. Cr(NO3)2(aq) + (NH4)½CO3(aq) -…
A:
Q: Reaction: Cu(NO3)2. + Na2SO4 Molecular equation: Net ionic equation:
A: Molecular equation contains the elements or compounds in molecular form. Net ionic equation…
Q: Q.1: For the following reaction : K3P04 (aq) + Al(NO3)3 (aq) | AIPO4 (s) + KNO3 (aq) a- Write the…
A: The balanced Molecular complete Reaction is that Reaction in which all reactants and products are…
Q: 2. Write ionic and net ionic equations for the following reactions: NaNO3 + KCl AgNO3 + Na2SO4…
A: (a)In the first three reactions, a product is being formed that is insoluble in water at moderate…
Q: Two aqueous solutions of Pb(NO3)2 and KI were mixed together in a test tube. a) Write the…
A:
Q: Ba(NO3)2(aq) + NH4Cl(aq) a. Full Molecular Equation: b. Full Ionic Equation: c. Net-Ionic…
A: Concept introduction: Molecular equation: It is the balanced chemical reaction in which the reacting…
Q: (a) Write chemical equations which show what happens when the following substances are mixed with…
A: Two questions based on inorganic equations.
Q: Generic Chemical Equation: 2A + 4B---> 3C What type of reaction the equation represents? A.…
A:
Q: - Which one of the following is the correct net ionic equation for the reaction that occurs when…
A: Molecular reaction equation between Pb(NO3)2 and KI : Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq…
Q: Pb(NO3)2 + NH4Cl = PbCl2 + NH4NO3 molecular and Ionic equations ?
A: In a chemical reaction, reactants react together to form product. Consider the given reaction.…
Q: Q.1: For the following reaction : K3P04 (aq) + Al(NO3)3 (aq) I AIP04 (s) + KNO3 (aq) a- Write the…
A:
Q: You react LiCl and AgNO3. What is the net ionic equation? Select one: a. Ag+(aq) + Cl-(aq) --> AgCl…
A: Aqueous Lithium chloride reacts with aqueous silver nitrate to produce an insoluble solid silver…
Q: (NH4)2SO4(aq) + Na2CO3= Balanced equation total ionic equation Net ionic equation
A: Balanced chemical equation of a reaction is written according to law of conservation of…
Q: Is the total ionic equation the same as the net ionic equation when Sr(OH)₂(aq) and H₂SO₄(aq) react?…
A: The ionic equation indicates all the species taking part in a chemical reaction, which is obtained…
Q: CuSO4 + 4NH3 -------> Cu(NH3)4 + SO4 written as a net ionic
A: We are given equation between ammonia solution and copper sulphate solution and we have to write…
Q: Ba(NO3)2(aq) + Na2SO4(aq) a. Full Molecular Equation: b. Full-Ionic Equation: c. Net-ionic…
A: This is possibly a double replacement/displacement reaction in which the cations or anions switch…
Q: Write the net ionic equation for the following molecular equation. Pb(NO,)2(aq) +…
A:
Q: 2. Consider the reaction Pb(NO:)2 + NazCO3 -→ ? + ? (a) Write the molecular equation (b) write the…
A: Spectator ions are that doesn't directly involve in the reaction or remain unchanged during the…
Q: _K2CO3 (aq) + _Mg(NO3)2 (aq) Complete Ionic Equation: + + + + + Net Ionic Equation: b) Molecular…
A: a) Molecular equation : K2CO3(aq) + Mg(NO3)2(aq) b) Molecular equation : H2SO4 (aq) + Li2CO3 (aq)…
Q: Molecular equation: HC2H302 (aq) + NH3 (aq) → NH4C2H3O2 (aq) Complete ionic equation: 4 Net ionic…
A:
Q: Example: a) molecular equation 3 NaOH(aq) + FeCl3{aq) → Fe(OH)3(5) + 3 NaCl(aq) b) total ionic…
A: NOTE: please note according to our policies , we can only answer one question at one time. you have…
Q: Write the balanced COMPLETE ionic equation for the reaction when AuBr3 and NH,NO, are mixed in…
A: Ionic Equation can be defined as the chemical equation in which different ions in the aqueous…
Q: eaction: Fe(NO3)3 + Na2SO4 Molecular equation: Net ionic equation:
A: The Molecular equation and Net ionic equation for the reaction -
Q: Q.2: For the following reaction : Na2SO4 (aq) + BaCl2 (aq) I BaSO4 (s) + NaCI (aq) a- Write the…
A: For balanced chemical equation no of atoms in reactant side must be equal to the no of atoms in…
Q: Reaction: Ca(NO3)2, + NaOH Molecular equation: Net ionic equation:
A: molecular equation: Ca(NO3)2(aq) + 2NaOH(aq)→Ca(OH)2(s) + 2NaNO3(aq)
Q: 4. Mg + Al(NO3)3 (aq) Molecular equation: Total ionic: Net ionic:
A:
Q: Example Overall equation: Pb(NO3)2(aq) + Na,SO4(aq) PBSO4(s) + 2NaNO3(aq) Write the net ionic…
A:
Q: Reactant 1 : 0.1M Pb(NO3)2 Reactant 2 : 0.1M NaI Equations Molecular equation : complete…
A:
Q: Sodium thiosulfate, Na2S2O3, is an important reagent for titrations. Its solutions can be…
A: Molarity of a solution is defined as the moles of solute present in one litre of solution.
Q: Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of…
A:
Q: Solve the attached problem
A: Aqueous manganese (II) nitrate is reacted with aqueous ammonia to form manganese hydroxide and…
Q: Reaction: Pb(NO3)2 + Na2CO3 Molecular equation: Net ionic equation:
A: Pb(NO3)2 - Lead nitrate Na2CO3 - Sodium carbonate PbCO3 -Lead carbonate NaNO3 - Sodium nitrate
Q: How do you write a net ionic equation for the formation of gas ex Al2(SO4)3(NH4)2SO4 •24 H2O +…
A:
Q: AgNO3(aq) + NH4Cl(aq) a. Full Molecular Equation: b. Full-Ionic Equation: c. Net-Ionic Equation:…
A: Silver nitrate (AgNO3(aq)) reacts with ammonium chloride (NH4Cl(aq)) to give the precipitate of…
Q: a) molecular equation 3 NaOH(aq) + FeCl3(aq) → F b) total ionic equation c) net ionic equation 1st…
A: Introduction: We are given with reaction:
Q: Write the molecular, complete, and net ionic reactions for aqueous lithium phosphate reacting with…
A: Molecular equation for the reaction of Lithium phosphate with gold nitrate take place as follows;…
Q: Reaction 3: Aqueous sodium phosphate + aqueous copper(II) sulfate Balanced Molecular Equation (from…
A: Correction: It should be Cu3(PO4)2(s) not Cu(PO4)2(s) in the product side. Given, The balanced…
Q: 3CoCl2 + 2Fe(NO3)3 = 3Co(NO3)2 + 2FeCl3 Write the complete ionic equation and net ionic equation.
A: A molecular equation represents molecules of reactants and products, a complete ionic equation…
Q: Suppose you are titrating a solution of sodium oxalate, which has a molar mass of 134 g/mol, with a…
A:
Q: 2 HC162) + Mg (S), Molealar equation Total jonic equation Net jonic equation
A:
Q: 4. Na,CrO4 + Fel3 a. Write the balanced chemical equation for the reaction. b. Write the total ionic…
A: Balancing equation: As per our guideline we have to answer first three questions only.
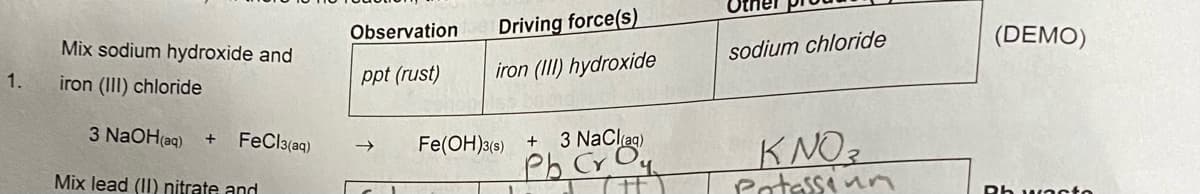

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

- 0.7050-g of pure KHP (FW = 204.2) was dissolved and titrated with 35.00mL of NaOH solution. The excess NaOH was backtitrated with 5.00mL of HCl solution. In a separate titration it was found that 30.00mL of NaOH will react with 28.00mL of HCl. Find the molarity of NaOH and HCl. Show all the solutions involved and round-off final answers to two decimal places except for Molarity. (Molar concentrations should be in four decimal places)and Employees CH STATE GE 90 ENDULERSE IS FOREVER "ON-Tame Name Jodi Rayal Molarity = wt. x1000 3. Calculate the massof Potassium hydrogen phthalate (KHP) to prepare a 250.0 mL solution of 0.1000 M KHP solution. Mol ut. x Voluse the 2500×1000 204.22250 mass Initial Buret Reading Final Buret Reading Vol NaOH Added Moles of NaOH Moles of HCI Volume of unknown HCI solution Concentration of HCI Average concentration of HCI of KHP = 5.11g 4. Complete the table below for the following neutralization reaction of an unknown concentration of hydrochloric acid with 0.100 M sodium hydroxide: Mu spate solution Trial 1 0 11.15 го data and calculate the concentration 15 Trial 2 11.15 22.15 Trial 3 22.15 33.25 abyh 067 LANDINUKASHU TROWTHCENTISSATE ARAD Ma That par މއކނި REMURNAR MISAMARALISERDRY TileTOPIC: GRAVIMETRY SHOW THE SOLUTION The mercury in a 0.7152-g sample was precipitated with an excess of paraperiodic acid, H5IO6, according to the following reactions:5 Hg+2 + 2 H5IO6 ---> Hg5(IO6)2(s) + 10 H+The precipitate was filtered, washed free of precipitating agent, dried and found to weigh 0.3408-g. Calculate the percentage of Hg2Cl2 in the sample. Molar Masses: Hg5(IO6)2 = 1448.75 Hg2Cl2 = 472.09 Answer: 38.82% Hg2Cl2 An iron ore was analyzed by dissolving a 1.1324-g sample in concentrated HCl. The resulting solution was diluted with water, and the iron (III) was precipitated as the hydrous oxide Fe2O3·xH20 by the addition of NH3. After filtration and washing, the residue was ignited at a high temperature to give 0.5394 g of pure Fe2O3. Calculate (a) % Fe, and (b) % Fe3O4 in the sampleMolar Masses: Fe2O3 = 159.69 Fe = 55.847 Fe3O4 = 231.54 Answer: 33.32% Fe and 46.04% Fe3O4
- When a Vitamin C (ascorbic acid; MM = 176.12 g mol-1) tablet is crushed, dissolved and titrated with 0.0340 M KIO3(aq) to a purple/blue endpoint (given by a starch indicator), the volume of KIO3 used is 29.80 mL. If 60 mg of ascorbic acid is the recommended dietary allowance (i.e., 100% of the RDA), then what is the % RDA for the Vitamin C in the tablet? KIO3(aq) + 5 KI + 6 H+ → 3 I2(aq) + 3 H2O I2 (aq) + ascorbic acid → 2 I- + dehydroascorbic acid1,5419 g of magnetite (Fe3O4) ore; in concentrated HCL to form a mixture of Fe + 2 and Fe+3it's unraveling. By adding HNO3 to it, all Fe+2 s are upgraded to Fe+3. And the addition of FE+3 s NH3,with Fe (OH)3, precipitating into. The sediment is weighed as 0.8525 g in the form of Fe2O3 after the necessary operations. Calculate the percentage of Fe3O4 in the sample.The mercury in a 0.8142-g sample was precipitated with an excess of paraperiodic acid, H5IO6: 5 Hg 2+ + 2 H5IO6 ---> Hg5(IO6)2 (s) + 10 H + The precipitate (MW = 1448.8 g/mol) was filtered, washed free of precipitating agent, dried, and weighed, and 0.4114 g was recovered. Calculate the a) % Hg (200.6 g/mol) b) % Hg2Cl2 (472.1 g/mol)
- Calculate the weight of pure sodium carbonate that is necessary to prepare 2.806 L of 0.223 N Na2CO3 (105.99 g/mol) from the primary-standard solid. Assume the solution is to be used for titrations in which the reaction is: CO32- + 2H+ ----> H2O + CO2 Express you answers in 3 decimal placesAspirin powder = 0.8110g MW of Aspirin = 180g.mol-1 Volume of 0.5N HCl consumed in back titration = 23.50mL Volume of 0.5N HCl consumed in blank titration = 44.50mL Percent purity (USP/NF) = Aspirin tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of aspirin (C9H8O4) What is the calculated weight (in grams) of pure aspirin?..What volume of 0.10 moldm-3 sulphuric acid would be required to neutralise a mixture of 1.06 g of anhydrous sodium carbonate and 4.00 g of sodium hydroxide? (H2SO4 + 2NaOH ---> Na2SO4 + 2H2O) and (H2SO4 + Na2CO3 ® ---> Na2SO4+ H2O + CO2)
- You collected the following data from a titration experiment using a 0.129M standardized NaOH solution to titrate a 26.55 mL solution with an unknown Molarity concentration (M) of sulfuric acid (H2SO4). Initial Burette Reading (mL) Final Burette Reading (mL) Vol Delivered (mL) Trial 1 0.44 19.69 ?? Trial 2 0.18 17.2 Trial 3 0.50 19.94 For just Trial 1, determine the amount of NaOH delivered for the titration with appropriate significant digits. Do not include units.A sample of pure sodium oxalate weighing 0.1050 g is ignited [Na2C2O4 (s) --> Na2CO3 (s) + CO (g)] and the resulting product requires 15.00 mL of a solution of H2SO4 for complete neutralization. What is the normality of the acid? MM of Na2CO3 = 106.0 g/molMM of Na2C2O4 = 134.0 g/molMM of CO = 28.01 g/molMM of H2SO4 = 98.08 g/molA weight of 0.50 g was taken impure container containing sodium carbonate and bicarbonate. Dissolved in water and then crushed with hydrochloric acid (0.1 N), the burette reading game was at the endpoint of phenolphthalein of 10.5 ml and at the end point of the orange methylation point 30.1 ml. The percentage of sodium carbonate was in ................. knowing that the weights are: Na: 23, C: 12, O: 16

