Exercise A: Amino Acid Functional Groups Figure 1 below shows one of the 20 amino acids that make up proteins. Recall that carbon can form four covalent bonds. Amino acids consist of a central carbon, called the a-carbon, that is bonded to four different chemical groups. H CH2 OH Figure 1. Structure of an amino acid Answer the below questions in your own document. • On the amino acid shown in Figure 1, label the a-carbon. • The a-carbon of each of the 20 amino acids is bonded to one hydrogen atom, one amino group, one carboxyl group, and one R group (more on that below). You should recognize the amino and carboxyl groups from our discussion of functional groups in organic molecules. Circle and label* the amino group and the carboxyl group in Figure 1. *Note: our goal in this question, and in similar questions throughout this lab, is for you to be able to identify specific structures. You can do this circling/labeling in whatever way is easiest for you. You might want to draw the structures on a piece of paper, or use a computer program (like Powerpoint, Photoshop, Paint, Preview, etc.) to draw on these images. Whatever is easiest for you! The last bond an a-carbon in an amino acid makes is to an R group.or side- chain. Circle and label the R group in Figure 1. The next page of this handout shows the structures of all 20 amino acids (Figure 2). They are categorized into 4 chemical groups: nonpolar, uncharged polar, acidic, and basic. • Using the three groups you identified in Figure 1 as a reference, what is the only thing that is different about each of the 20 amino acids?
Exercise A: Amino Acid Functional Groups Figure 1 below shows one of the 20 amino acids that make up proteins. Recall that carbon can form four covalent bonds. Amino acids consist of a central carbon, called the a-carbon, that is bonded to four different chemical groups. H CH2 OH Figure 1. Structure of an amino acid Answer the below questions in your own document. • On the amino acid shown in Figure 1, label the a-carbon. • The a-carbon of each of the 20 amino acids is bonded to one hydrogen atom, one amino group, one carboxyl group, and one R group (more on that below). You should recognize the amino and carboxyl groups from our discussion of functional groups in organic molecules. Circle and label* the amino group and the carboxyl group in Figure 1. *Note: our goal in this question, and in similar questions throughout this lab, is for you to be able to identify specific structures. You can do this circling/labeling in whatever way is easiest for you. You might want to draw the structures on a piece of paper, or use a computer program (like Powerpoint, Photoshop, Paint, Preview, etc.) to draw on these images. Whatever is easiest for you! The last bond an a-carbon in an amino acid makes is to an R group.or side- chain. Circle and label the R group in Figure 1. The next page of this handout shows the structures of all 20 amino acids (Figure 2). They are categorized into 4 chemical groups: nonpolar, uncharged polar, acidic, and basic. • Using the three groups you identified in Figure 1 as a reference, what is the only thing that is different about each of the 20 amino acids?
Biochemistry
9th Edition
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Chapter3: Amino Acids And Peptides
Section: Chapter Questions
Problem 13RE: MATHEMATICAL An organic chemist is generally happy with 95% yields. If you synthesized a polypeptide...
Related questions
Question

Transcribed Image Text:Exercise A: Amino Acid Functional Groups
Figure 1 below shows one of the 20 amino acids that make up proteins. Recall that
carbon can form four covalent bonds. Amino acids consist of a central carbon, called
the a-carbon, that is bonded to four different chemical groups.
H
+
CH2
OH
Figure 1. Structure of an amino acid
Answer the below questions in your own document.
• On the amino acid shown in Figure 1, label the a-carbon.
• The a-carbon of each of the 20 amino acids is bonded to one hydrogen
atom, one amino group, one carboxyl group, and one R group (more on
that below). You should recognize the amino and carboxyl groups from our
discussion of functional groups in organic molecules. Circle and label* the
amino group and the carboxyl group in Figure 1.
*Note: our goal in this question, and in similar questions throughout this lab, is for
you to be able to identify specific structures. You can do this circling/labeling in
whatever way is easiest for you. You might want to draw the structures on a piece of
paper, or use a computer program (like Powerpoint, Photoshop, Paint, Preview, etc.)
to draw on these images. Whatever is easiest for you!
• The last bond an a-carbon in an amino acid makes is to an R group.or side-
chain. Circle and label the R group in Figure 1.
The next page of this handout shows the structures of all 20 amino acids (Figure 2).
They are categorized into 4 chemical groups: nonpolar, uncharged polar, acidic, and
basic.
• Using the three groups you identified in Figure 1 as a reference, what is the only
thing that is different about each of the 20 amino acids?
Look at the amino acids in each of the four groups and compare them to the ones in
the other groups. Figure out rules that describe what the members of each group
Screenshot
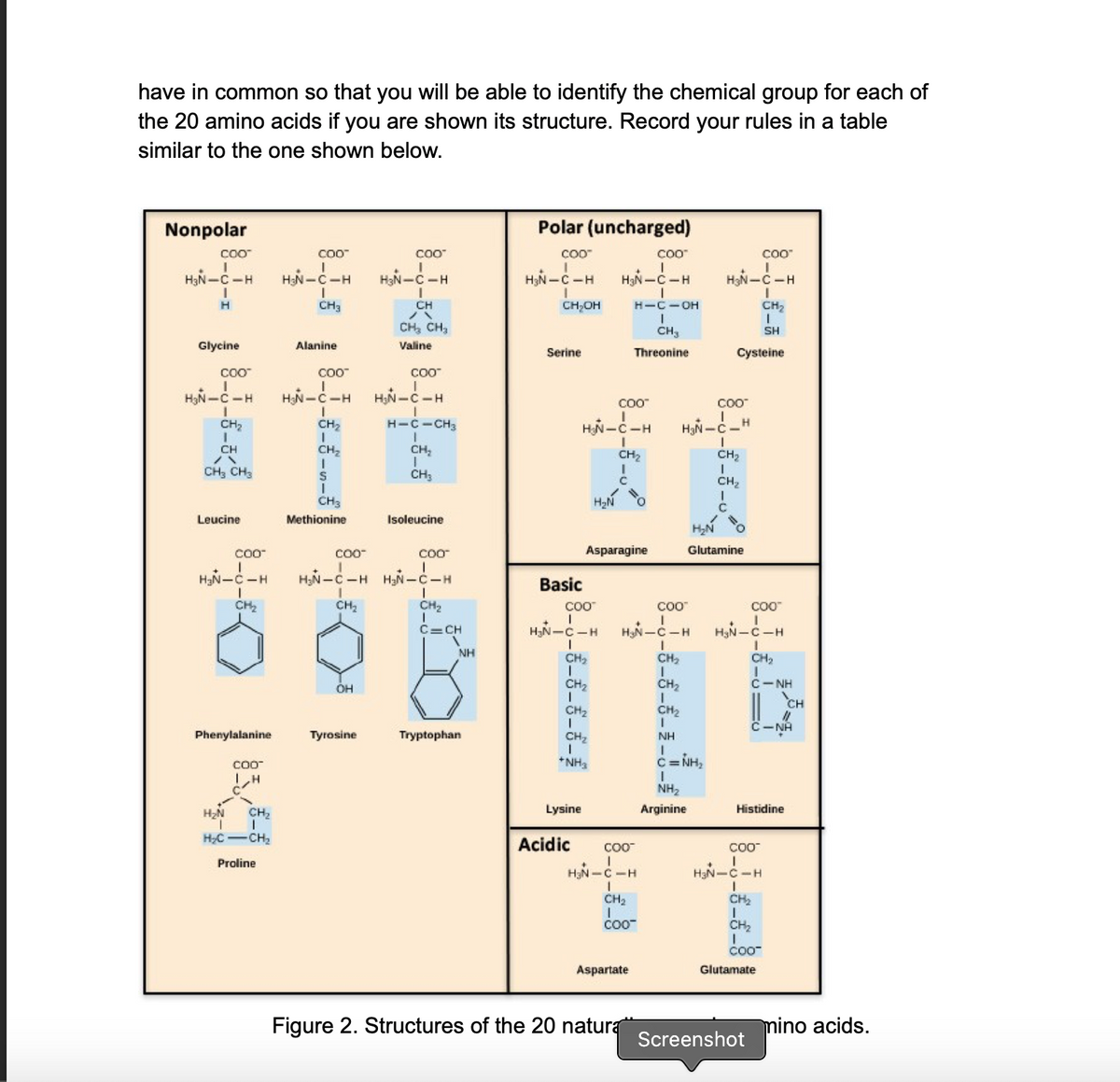
Transcribed Image Text:have in common so that you will be able to identify the chemical group for each of
the 20 amino acids if you are shown its structure. Record your rules in a table
similar to the one shown below.
Nonpolar
Polar (uncharged)
COO
COO
COO"
H3N-C-H
HN-C-H
HạN-C -H
H3N -C -H
HaN-C-H
H3N-C-H
H
CH3
CH
バ
CH,OH
H-C-OH
CH2
CH, CH,
CH3
SH
Glycine
Alanine
Valine
Serine
Threonine
Cysteine
COO
COO
COO
HaN-C-H
HN-C-H
HN-C-H
COO
CH2
CH2
H-C-CH3
HN-C-H
CH
CH2
CH,
CH2
CH2
CH; CH3
CH3
CH2
CH3
H2N
Leucine
Methionine
Isoleucine
Coo
COo-
Coo
Asparagine
Glutamine
H3N-C-H
HN-C -H HN-c-H
Basic
CH2
CH2
CH2
COO
CO"
C=CH
HN-C-H
-C-H
-C-H
NH
CH,
CH2
CH2
ÓH
CH2
CH2
C-NH
CH
CH2
CH2
C-NA
Phenylalanine
Tyrosine
Tryptophan
CH2
NH
CO-
*NH3
C3D
NH2
CH,
Lysine
Arginine
Histidine
HC CH2
Acidic
COO
CO0
Proline
HN-C-H
HạN-C-H
CH2
CH2
COO
CH2
CO0
Aspartate
Glutamate
Figure 2. Structures of the 20 natura
mino acids.
Screenshot
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Understanding Nutrition (MindTap Course List)
Health & Nutrition
ISBN:
9781337392693
Author:
Eleanor Noss Whitney, Sharon Rady Rolfes
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Understanding Nutrition (MindTap Course List)
Health & Nutrition
ISBN:
9781337392693
Author:
Eleanor Noss Whitney, Sharon Rady Rolfes
Publisher:
Cengage Learning