Experiment 10-Polarity and Solubility name section date Worksheet I. Predictions of Polarity and Solubility Name Lewis Structure Polarity Soluble in C6H12? Soluble in H20? Water H-O-H нн Н C. Cyclohexane (C6H12) H H-C С-н Н-с C-H н HHH H H 1. Ethanol H-C-C-O-H D нн Н H O H 2. Isopropanol Н-С-с-с-Н ннн H H H H 3. Butanol Н-С-с-с-с-о-н нннн H o H 4. Acetone Н-с-с-с-н Н Н-с-с-о-н 5. Acetic acid Н :0, н Н-с-с-о-с-с-н H H 6. Ethyl acetate H H Н 0 с-о-с-н 4 7. Wintergreen Н Н H-C-H I H H C н 8. Xylene нСсес H H H 9. Sodium chloride H:0 I I H-C-C-O Na Н 10. Sodium acetate 131 H-CIH I HIC I I
Experiment 10-Polarity and Solubility name section date Worksheet I. Predictions of Polarity and Solubility Name Lewis Structure Polarity Soluble in C6H12? Soluble in H20? Water H-O-H нн Н C. Cyclohexane (C6H12) H H-C С-н Н-с C-H н HHH H H 1. Ethanol H-C-C-O-H D нн Н H O H 2. Isopropanol Н-С-с-с-Н ннн H H H H 3. Butanol Н-С-с-с-с-о-н нннн H o H 4. Acetone Н-с-с-с-н Н Н-с-с-о-н 5. Acetic acid Н :0, н Н-с-с-о-с-с-н H H 6. Ethyl acetate H H Н 0 с-о-с-н 4 7. Wintergreen Н Н H-C-H I H H C н 8. Xylene нСсес H H H 9. Sodium chloride H:0 I I H-C-C-O Na Н 10. Sodium acetate 131 H-CIH I HIC I I
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 107QRT
Related questions
Question
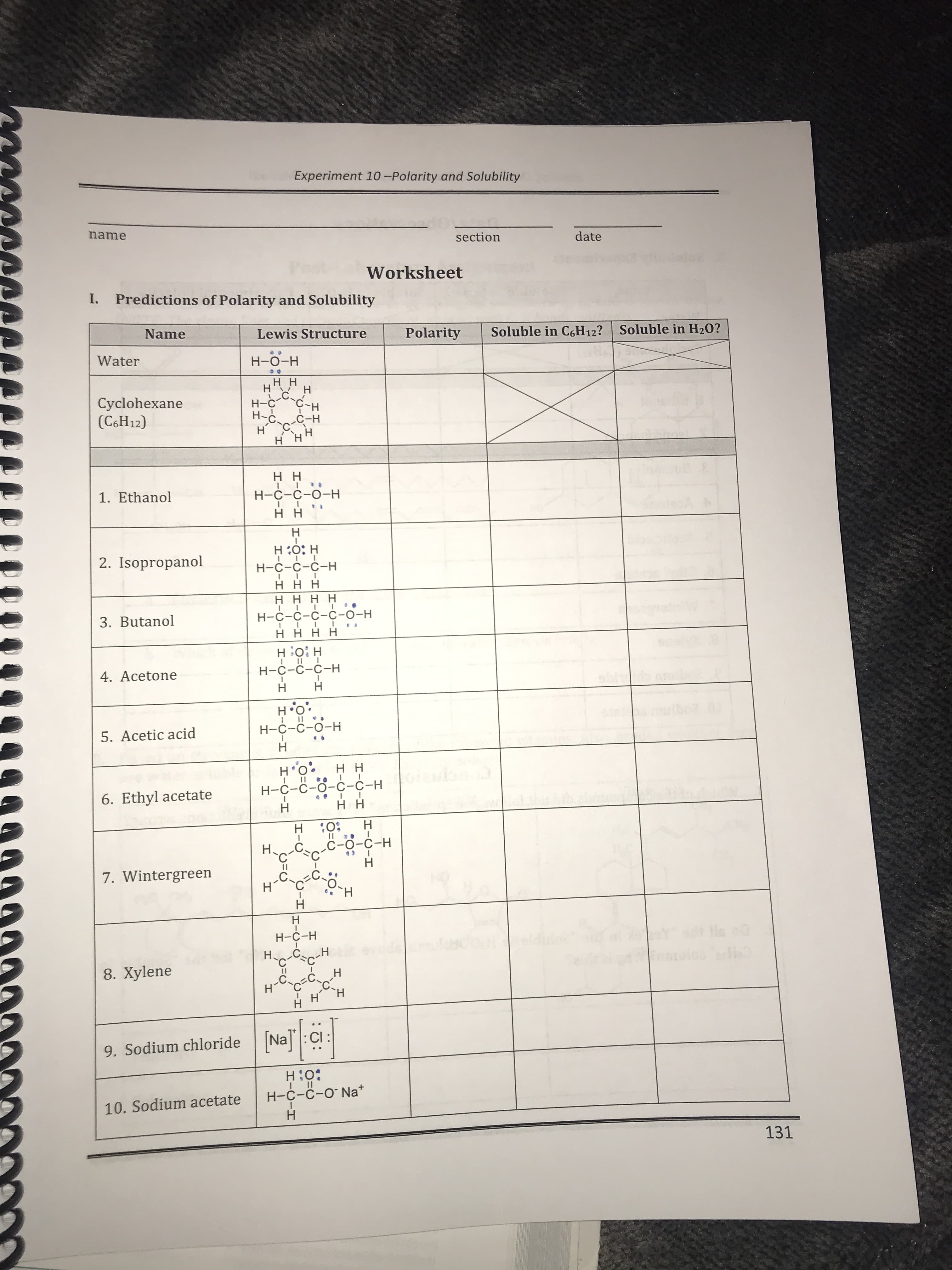
Transcribed Image Text:Experiment 10-Polarity and Solubility
name
section
date
Worksheet
I.
Predictions of Polarity and Solubility
Name
Lewis Structure
Polarity
Soluble in C6H12?
Soluble in H20?
Water
H-O-H
нн
Н
C.
Cyclohexane
(C6H12)
H
H-C
С-н
Н-с
C-H
н
HHH
H H
1. Ethanol
H-C-C-O-H
D
нн
Н
H O H
2. Isopropanol
Н-С-с-с-Н
ннн
H H H H
3. Butanol
Н-С-с-с-с-о-н
нннн
H o H
4. Acetone
Н-с-с-с-н
Н
Н-с-с-о-н
5. Acetic acid
Н
:0, н
Н-с-с-о-с-с-н
H H
6. Ethyl acetate
H H
Н
0
с-о-с-н
4
7. Wintergreen
Н
Н
H-C-H
I
H H
C
н
8. Xylene
нСсес
H
H
H
9. Sodium chloride
H:0
I I
H-C-C-O Na
Н
10. Sodium acetate
131
H-CIH
I
HIC
I
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning