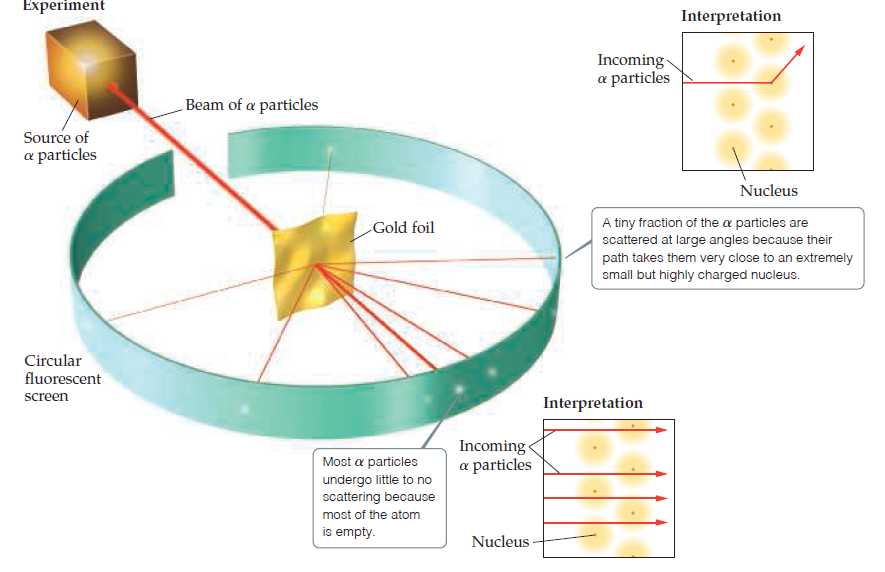
Experiment Interpretation Incoming a particles Beam of a particles Source of a particles Nucleus A tiny fraction of the a particles are scattered at large angles because their path takes them very close to an extremely small but highly charged nucleus. Gold foil Circular fluorescent screen Interpretation Incoming a particles Most a particles undergo little to no scattering because most of the atom is empty. Nucleus A Figure 2.9 Rutherford's a-scattering experiment. When a particles pass through a gold foil, most pass through undeflected but some are scattered, a few at very large angles. According to the plum-pudding model of the atom, the particles should experience only very minor deflections. The nuclear model of the atom explains why a few a particles are deflected at large angles. Although the nuclear atom has been depicted here as a yellow sphere, it is important to realize that most of the space around the nucleus contains only the low-mass electrons.
Experiment Interpretation Incoming a particles Beam of a particles Source of a particles Nucleus A tiny fraction of the a particles are scattered at large angles because their path takes them very close to an extremely small but highly charged nucleus. Gold foil Circular fluorescent screen Interpretation Incoming a particles Most a particles undergo little to no scattering because most of the atom is empty. Nucleus A Figure 2.9 Rutherford's a-scattering experiment. When a particles pass through a gold foil, most pass through undeflected but some are scattered, a few at very large angles. According to the plum-pudding model of the atom, the particles should experience only very minor deflections. The nuclear model of the atom explains why a few a particles are deflected at large angles. Although the nuclear atom has been depicted here as a yellow sphere, it is important to realize that most of the space around the nucleus contains only the low-mass electrons.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 6RQ: Consider Ernest Rutherfords -particle bombardment experiment illustrated in Fig. l- 16. How did the...
Related questions
Question
Go Figure What is the charge on the particles that form the beam? Will they be
attracted to or repelled from the positively charged gold nuclei?
Transcribed Image Text:Experiment
Interpretation
Incoming
a particles
Beam of a particles
Source of
a particles
Nucleus
A tiny fraction of the a particles are
scattered at large angles because their
path takes them very close to an extremely
small but highly charged nucleus.
Gold foil
Circular
fluorescent
screen
Interpretation
Incoming
a particles
Most a particles
undergo little to no
scattering because
most of the atom
is empty.
Nucleus

Transcribed Image Text:A Figure 2.9 Rutherford's a-scattering experiment. When a particles pass through a gold foil,
most pass through undeflected but some are scattered, a few at very large angles. According
to the plum-pudding model of the atom, the particles should experience only very minor
deflections. The nuclear model of the atom explains why a few a particles are deflected at large
angles. Although the nuclear atom has been depicted here as a yellow sphere, it is important to
realize that most of the space around the nucleus contains only the low-mass electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning