Figure 13.9 Relation between solubility and temperature for several ionic compoundss. 100 Most ionic compounds 90 are more soluble at NANO3 higher temperatures. 80 70 60 KCI 50 NaCI 40 30 KCIОЗ 20 10 Ce2(SO4)3 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Experimental Procedure: Personal Protective Equipment (PPE) Required Safety glasses Full coverage pants Shoes which cover the entire foot 32 Solubility (g solute/100 g H2O) KNO3 Chemical Safety Potassium Nitrate is hazardous in case of skin contact (irritant), eye contact (irritant), inhalation (lung irritant), and ingestion. It is an oxidizer. Obtain a 50 mL Erlenmeyer flask and into it weigh between 11.5 and 12.5 grams of potassium nitrate from the reagent desk. Record the actual mass of the KNO3 only. For trial 1, measure out 10 mL of distilled water using your 10 mL graduated cylinder. Add this water to the flask and record the mass of the water only. Set up your Bunsen burner under a piece of wire gauze on the ring stand and gently warm the flask using a small blue flame heating the flask with an orange/yellow flame will deposit soot on the flask. Swirl the solution while heating using your tongs to hold the flask. As Soon as the KNO; is completely dissolved or the solution starts to boil (whichever comes first), remove the flask from the heat and turn off your Bunsen burner. Holding a thermometer in the solution, swirl the solution gently. Record the temperature at which fine crystals of solid just begin to appear in the solution. You may ignore any solid matter that did not dissolve readily when you heated the solution. For trial 2, add 3 mL more water to the solution from trial 1. Record the total mass of water in this solution and repeat the experiment as in trial 1. For trials 3 and 4, add 3 mL more water each time and repeat as before. From the data calculate the concentration of each solution in grams of solute per 100 grams of water. Plot these concentralions against temperature using a computer graphing/spreadsheet program such as MS Excel to draw the solubility curve of KNO3. If a graphing program is used, the points can be connected with a smoothed line (do not fit them to a linear trendline). Print out the graph and label the areas on either side of the curve (dilute solution, saturated solution and excess solute). On the same graph, plot the following literature values for the solubility of KNO3 and see how well they fit your curve. 40° 50° 70° 60° 30° Temperature: 20° KNO; solubility: 85.5 110 45.8 63.9 31.6 138 (g KNO3 / 100 g H20) Conclusion section. Write a coherent paragraph that addresses the following: Does KNO3 have an endothermic or exothermic heat of solution? Justify your answer based on your experimental evidence. Do the literature solubilities shown above lie on your experimental solubility curve? If not, use the direction of the error in your solubility curve (whether it is above or below the literature values) propose one or more likely sources of error - consider possible changes in (or errors in measuring) masses of water and KNO3 as well as the detection of the saturation temperature.
Figure 13.9 Relation between solubility and temperature for several ionic compoundss. 100 Most ionic compounds 90 are more soluble at NANO3 higher temperatures. 80 70 60 KCI 50 NaCI 40 30 KCIОЗ 20 10 Ce2(SO4)3 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Experimental Procedure: Personal Protective Equipment (PPE) Required Safety glasses Full coverage pants Shoes which cover the entire foot 32 Solubility (g solute/100 g H2O) KNO3 Chemical Safety Potassium Nitrate is hazardous in case of skin contact (irritant), eye contact (irritant), inhalation (lung irritant), and ingestion. It is an oxidizer. Obtain a 50 mL Erlenmeyer flask and into it weigh between 11.5 and 12.5 grams of potassium nitrate from the reagent desk. Record the actual mass of the KNO3 only. For trial 1, measure out 10 mL of distilled water using your 10 mL graduated cylinder. Add this water to the flask and record the mass of the water only. Set up your Bunsen burner under a piece of wire gauze on the ring stand and gently warm the flask using a small blue flame heating the flask with an orange/yellow flame will deposit soot on the flask. Swirl the solution while heating using your tongs to hold the flask. As Soon as the KNO; is completely dissolved or the solution starts to boil (whichever comes first), remove the flask from the heat and turn off your Bunsen burner. Holding a thermometer in the solution, swirl the solution gently. Record the temperature at which fine crystals of solid just begin to appear in the solution. You may ignore any solid matter that did not dissolve readily when you heated the solution. For trial 2, add 3 mL more water to the solution from trial 1. Record the total mass of water in this solution and repeat the experiment as in trial 1. For trials 3 and 4, add 3 mL more water each time and repeat as before. From the data calculate the concentration of each solution in grams of solute per 100 grams of water. Plot these concentralions against temperature using a computer graphing/spreadsheet program such as MS Excel to draw the solubility curve of KNO3. If a graphing program is used, the points can be connected with a smoothed line (do not fit them to a linear trendline). Print out the graph and label the areas on either side of the curve (dilute solution, saturated solution and excess solute). On the same graph, plot the following literature values for the solubility of KNO3 and see how well they fit your curve. 40° 50° 70° 60° 30° Temperature: 20° KNO; solubility: 85.5 110 45.8 63.9 31.6 138 (g KNO3 / 100 g H20) Conclusion section. Write a coherent paragraph that addresses the following: Does KNO3 have an endothermic or exothermic heat of solution? Justify your answer based on your experimental evidence. Do the literature solubilities shown above lie on your experimental solubility curve? If not, use the direction of the error in your solubility curve (whether it is above or below the literature values) propose one or more likely sources of error - consider possible changes in (or errors in measuring) masses of water and KNO3 as well as the detection of the saturation temperature.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.6QP: What is the usual solubility behavior of an ionic compound in water when the temperature is raised?...
Related questions
Question
Can an aqueous solution be prepared that is 30% by mass KNO3 at 20 °C? (Hint: use the data from the literature values of the solubility of potassium nitrate.)
Answer choices
yes it can be made b/c 30 g KNO3/100 g H2O is less than 31. 6 g KNO3/100 g H2O
No it can't be made b/c 30 g KNO3/100 g H2O is less than 31. 6 70.38 g KNO3/100 g H2O
yes it can be made b/c 30 g KNO3/100 g H2O is less than 45.8 g KNO3/100 g H2O
No it can't be made b/c 30 g KNO3/100 g H2O is less than 45.8 g KNO3/100 g H2O
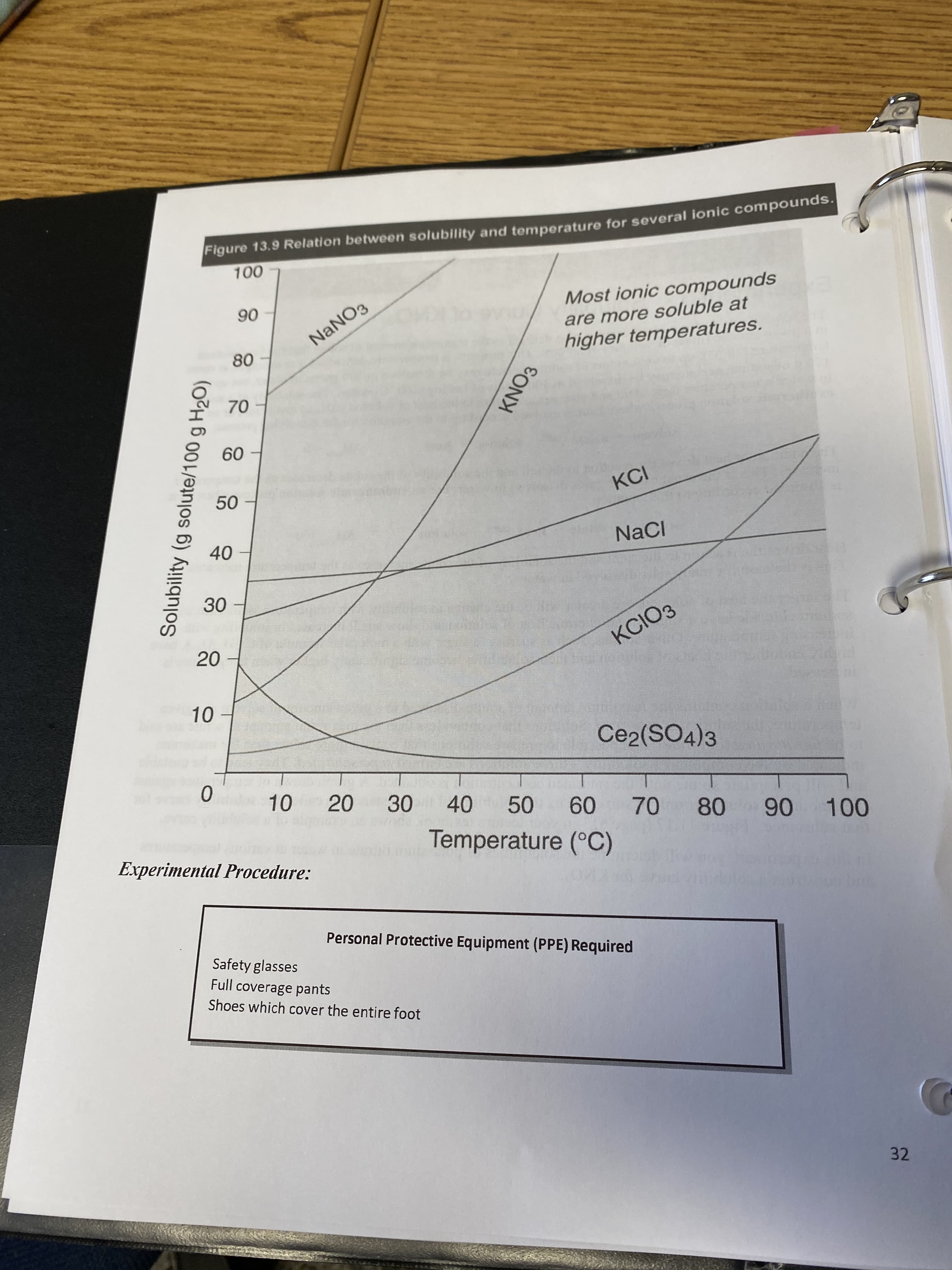
Transcribed Image Text:Figure 13.9 Relation between solubility and temperature for several ionic compoundss.
100
Most ionic compounds
90
are more soluble at
NANO3
higher temperatures.
80
70
60
KCI
50
NaCI
40
30
KCIОЗ
20
10
Ce2(SO4)3
10 20
30
40 50
60 70
80 90 100
Temperature (°C)
Experimental Procedure:
Personal Protective Equipment (PPE) Required
Safety glasses
Full coverage pants
Shoes which cover the entire foot
32
Solubility (g solute/100 g H2O)
KNO3

Transcribed Image Text:Chemical Safety
Potassium Nitrate is hazardous in case of skin contact (irritant), eye
contact (irritant), inhalation (lung irritant), and ingestion. It is an oxidizer.
Obtain a 50 mL Erlenmeyer flask and into it weigh between 11.5 and 12.5 grams of potassium nitrate from
the reagent desk. Record the actual mass of the KNO3 only.
For trial 1, measure out 10 mL of distilled water using your 10 mL graduated cylinder. Add this water to the
flask and record the mass of the water only. Set up your Bunsen burner under a piece of wire gauze on the
ring stand and gently warm the flask using a small blue flame heating the flask with an orange/yellow
flame will deposit soot on the flask. Swirl the solution while heating using your tongs to hold the flask. As
Soon as the KNO; is completely dissolved or the solution starts to boil (whichever comes first), remove the
flask from the heat and turn off your Bunsen burner. Holding a thermometer in the solution, swirl the
solution gently. Record the temperature at which fine crystals of solid just begin to appear in the solution.
You may ignore any solid matter that did not dissolve readily when you heated the solution.
For trial 2, add 3 mL more water to the solution from trial 1. Record the total mass of water in this solution
and repeat the experiment as in trial 1. For trials 3 and 4, add 3 mL more water each time and repeat as
before.
From the data calculate the concentration of each solution in grams of solute per 100 grams of water.
Plot these concentralions against temperature using a computer graphing/spreadsheet program such as MS
Excel to draw the solubility curve of KNO3. If a graphing program is used, the points can be connected with
a smoothed line (do not fit them to a linear trendline). Print out the graph and label the areas on either side
of the curve (dilute solution, saturated solution and excess solute).
On the same graph, plot the following literature values for the solubility of KNO3 and see how well they fit
your curve.
40°
50°
70°
60°
30°
Temperature:
20°
KNO; solubility:
85.5
110
45.8
63.9
31.6
138
(g KNO3 / 100 g H20)
Conclusion section. Write a coherent paragraph that addresses the following:
Does KNO3 have an endothermic or exothermic heat of solution? Justify your answer based on
your experimental evidence.
Do the literature solubilities shown above lie on your experimental solubility curve? If not, use
the direction of the error in your solubility curve (whether it is above or below the literature values)
propose one or more likely sources of error - consider possible changes in (or errors in measuring)
masses of water and KNO3 as well as the detection of the saturation temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning