Figure E21.22 shows the bonding of cytosine and guanine. The O—H and H—N distances are each 0.110 nm. In this case, assume that the bonding is due only to the forces along the O—H—O, N—H—N, and O—H—N combinations, and assume also that these three combinations are parallel to each other. Calculate the net force that cytosine exerts on guanine due to the preceding three combinations. Is this force attractive or repulsive?
Figure E21.22 shows the bonding of cytosine and guanine. The O—H and H—N distances are each 0.110 nm. In this case, assume that the bonding is due only to the forces along the O—H—O, N—H—N, and O—H—N combinations, and assume also that these three combinations are parallel to each other. Calculate the net force that cytosine exerts on guanine due to the preceding three combinations. Is this force attractive or repulsive?
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter12: Fluid Dynamics And Its Biological And Medical Applications
Section: Chapter Questions
Problem 64PE: Oxygen reaches the veinless cornea of the eye by diffusing through its tear layer, which is 0.500-mm...
Related questions
Question
Figure E21.22 shows the bonding of cytosine and guanine. The O—H and H—N distances are each 0.110 nm. In this case, assume that the bonding is due only to the forces along the O—H—O, N—H—N, and O—H—N combinations, and assume also that these three combinations are parallel to each other. Calculate the
net force that cytosine exerts on guanine due to the preceding three combinations. Is this force attractive or repulsive?
Help please. I have been stuck for hours now
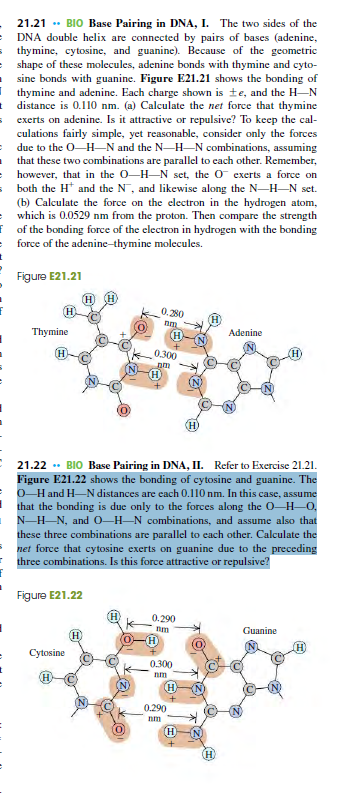
Transcribed Image Text:21.21 . BIO Base Pairing in DNA, I. The two sides of the
DNA double helix are connected by pairs of bases (adenine,
s thymine, cytosine, and guanine). Because of the geometric
e shape of these molecules, adenine bonds with thymine and cyto-
sine bonds with guanine. Figure E21.21 shows the bonding of
Ithymine and adenine. Each charge shown is te, and the H-N
t distance is 0.110 nm. (a) Calculate the net force that thymine
sexerts on adenine. Is it attractive or repulsive? To keep the cal-
culations fairly simple, yet reasonable, consider only the forces
= due to the O-H–N and the N-H–N combinations, assuming
that these two combinations are parallel to cach other. Remember,
however, that in the 0-H-N set, the O exerts a force on
both the H* and the N¯, and likewise along the N-H-N set.
(b) Calculate the force on the electron in the hydrogen atom,
which is 0.0529 nm from the proton. Then compare the strength
F of the bonding force of the electron in hydrogen with the bonding
force of the adenine-thymine molecules.
Figure E21.21
H
0.280
Thymine
(H)
Adenine
(H
0.300
(H)
pm
H)
21.22 . BIO Base Pairing in DNA, II. Refer to Exercise 21.21.
Figure E21.22 shows the bonding of cytosine and guanine. The
O-Hand H-N distances are each 0.110 nm. In this case, assume
that the bonding is due only to the forces along the O-H–0,
N-H-N, and 0–H–N combinations, and assume also that
these three combinations are parallel to cach other. Calculate the
net force that cytosine exerts on guanine due to the preceding
three combinations. Is this force attractive or repulsive?
Figure E21.22
0.290
Guanine
H)
Cytosine
0.300
nm
0.290
nm
H
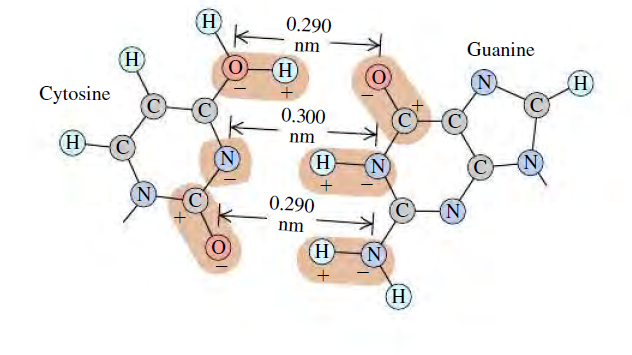
Transcribed Image Text:H
0.290
nm
Guanine
(H
H
H)
Cytosine
0.300
H)
nm
H
(N)
(N
0.290
(N
nm
H
N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning