Find the change in the internal energy of the gas as it moves from c to d. The change in the internal energy of the gas as it moves from a to c is -100 J and 90 J of heat energy must be transferred to the gas during the process c to d. An additional transfer of 40 J to it as heat is needed as it moves from d to a P а d C V (а) 60 J (b) -30J (c) 50 J (d) -50 J
Find the change in the internal energy of the gas as it moves from c to d. The change in the internal energy of the gas as it moves from a to c is -100 J and 90 J of heat energy must be transferred to the gas during the process c to d. An additional transfer of 40 J to it as heat is needed as it moves from d to a P а d C V (а) 60 J (b) -30J (c) 50 J (d) -50 J
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 80AP: A car tile contains 0.0380 m3 of air at a pressure of 2.20105 Pa (about 32 psi). How much more...
Related questions
Question
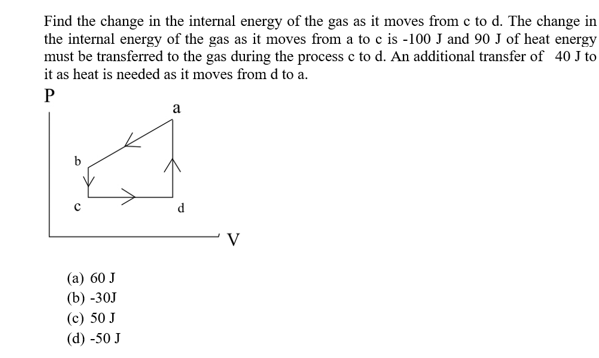
Transcribed Image Text:Find the change in the internal energy of the gas as it moves from c to d. The change in
the internal energy of the gas as it moves from a to c is -100 J and 90 J of heat energy
must be transferred to the gas during the process c to d. An additional transfer of 40 J to
it as heat is needed as it moves from d to a
P
а
d
C
V
(а) 60 J
(b) -30J
(c) 50 J
(d) -50 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning