For a hydrogen-like atom, classify the electron transitions according to whether they result in the absorption or emission of light. Absorption Emission Answer Bank n = 2 to n = 1 n = 1 to n = 3 n = 3 to n = 2 n = 3 to n = 5 Ignoring sign, which transition is associated with the greatest energy change? On = 2 to n = 1 On = 3 to n = 2 25 A étv w MacBook Air 80 888 F3 DII DD F4 F5 F6 F7 F8
For a hydrogen-like atom, classify the electron transitions according to whether they result in the absorption or emission of light. Absorption Emission Answer Bank n = 2 to n = 1 n = 1 to n = 3 n = 3 to n = 2 n = 3 to n = 5 Ignoring sign, which transition is associated with the greatest energy change? On = 2 to n = 1 On = 3 to n = 2 25 A étv w MacBook Air 80 888 F3 DII DD F4 F5 F6 F7 F8
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.21QP: Of the following possible transitions of an electron in a hydrogen atom, which emits light of the...
Related questions
Question
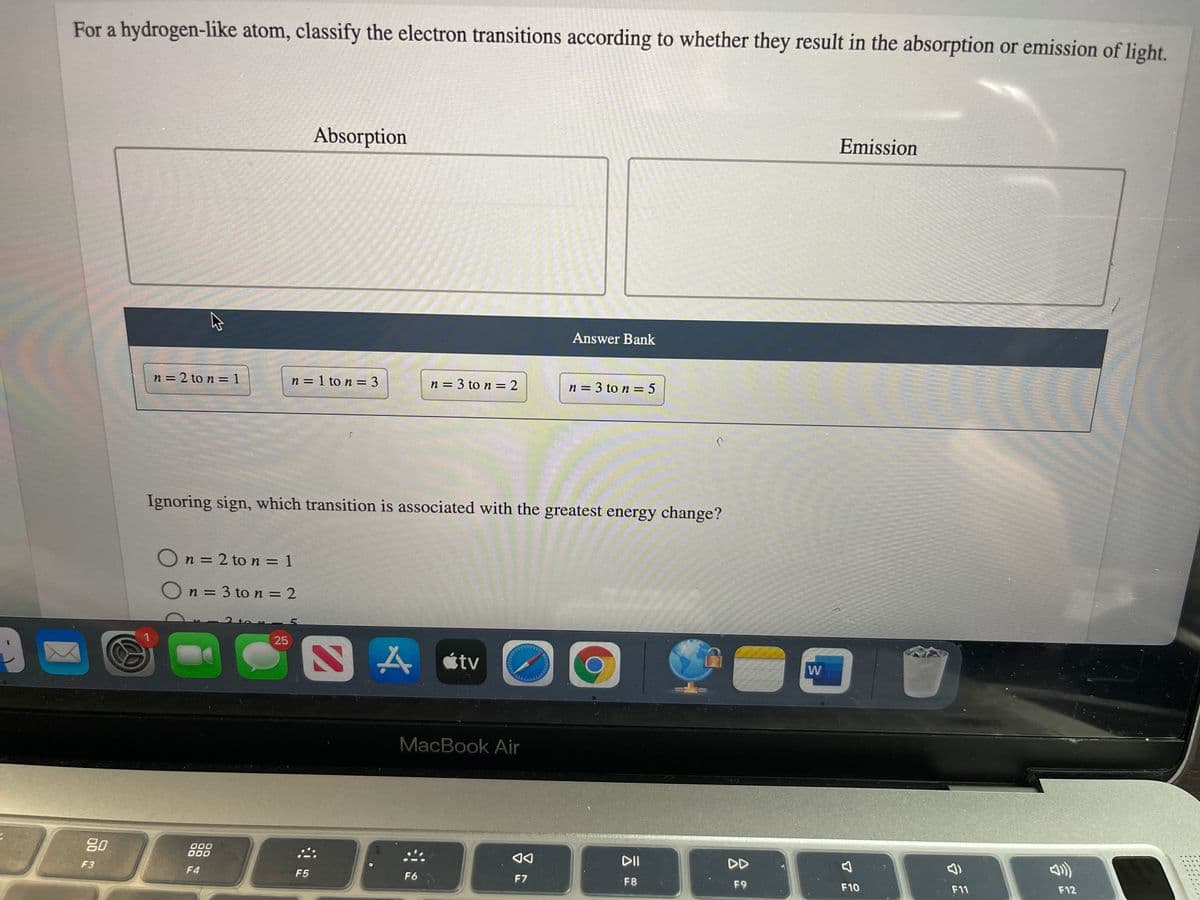
Transcribed Image Text:For a hydrogen-like atom, classify the electron transitions according to whether they result in the absorption or emission of light.
Absorption
Emission
Answer Bank
n = 2 to n = 1
n = 1 to n = 3
n = 3 to n = 2
n = 3 to n = 5
Ignoring sign, which transition is associated with the greatest energy change?
n = 2 to n = 1
On = 3 to n = 2
2 to
25
étv
W
MacBook Air
80
000
O00
F3
DII
DD
F4
F5
F6
F7
F8
F9
F10
F11
F12
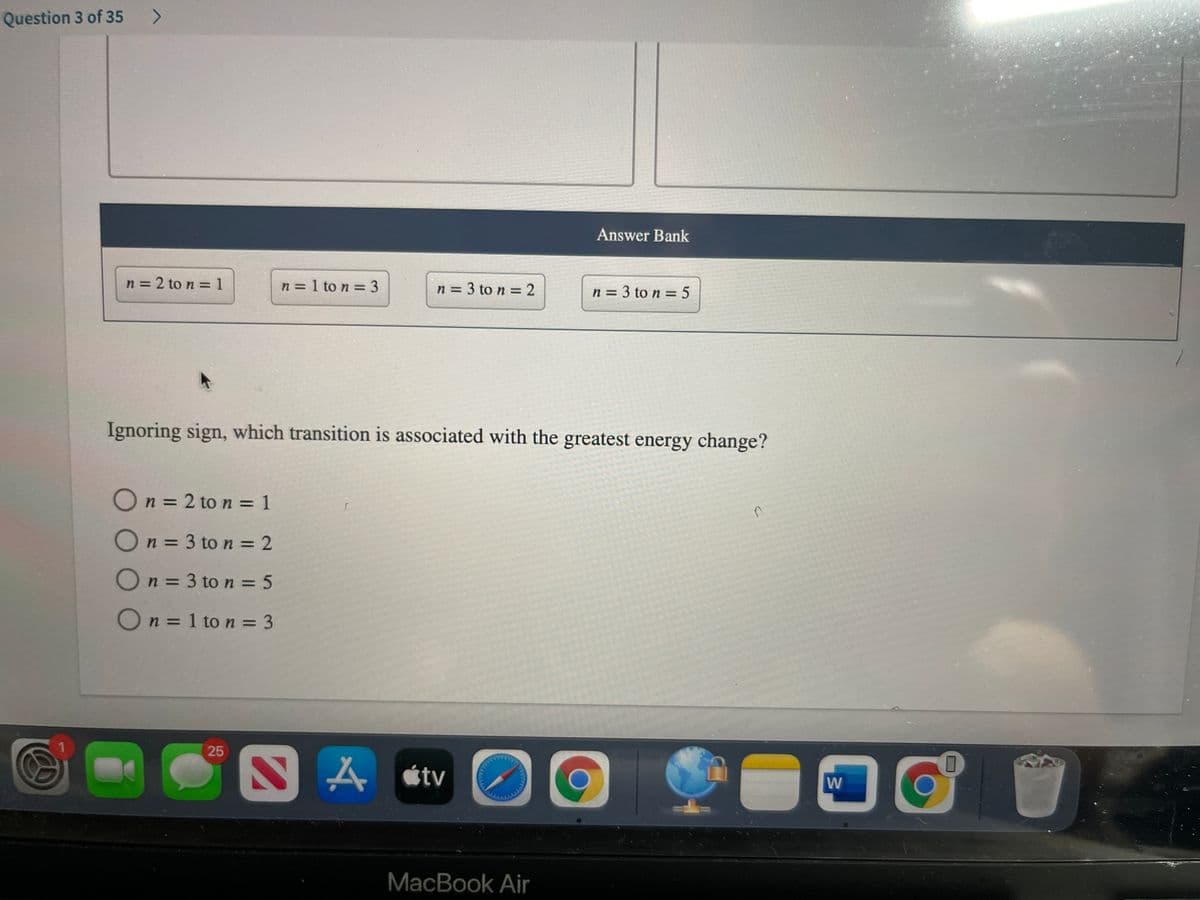
Transcribed Image Text:Question 3 of 35
<>
Answer Bank
n = 2 to n =1
n = 1 to n = 3
n = 3 to n = 2
n = 3 to n = 5
Ignoring sign, which transition is associated with the greatest energy change?
On = 2 to n = 1
On = 3 to n = 2
n3D
%3D
On= 3 to n = 5
On = 1 to n = 3
25
S A stv
W
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning