For each of the following species, determine (a) the electron geometry and (b) the hybridization for all nonhydrogen atoms. (i) C2H (v) CH;0* (ii) C2H5 (vi) C3H3 (all hydrogens are on the same carbon) (iii) CH2=0H+ (iv) CH&N*
For each of the following species, determine (a) the electron geometry and (b) the hybridization for all nonhydrogen atoms. (i) C2H (v) CH;0* (ii) C2H5 (vi) C3H3 (all hydrogens are on the same carbon) (iii) CH2=0H+ (iv) CH&N*
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 74AP: trans-tetrazene (N4H4) consists of a chain of four nitrogen atoms with each of the two end atoms...
Related questions
Question
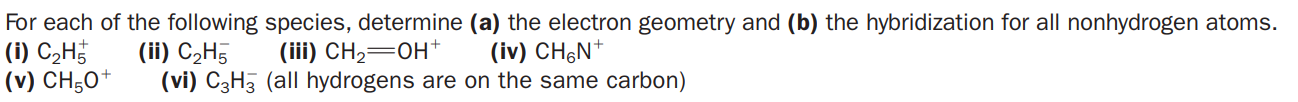
Transcribed Image Text:For each of the following species, determine (a) the electron geometry and (b) the hybridization for all nonhydrogen atoms.
(i) C2H
(v) CH;0*
(ii) C2H5
(vi) C3H3 (all hydrogens are on the same carbon)
(iii) CH2=0H+
(iv) CH&N*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning