For each pair of compounds listed, check the box next to the one with the higher boiling point. compounds higher boiling point Xe Rn GeCl GeH, '4. Sil, SiCl,
For each pair of compounds listed, check the box next to the one with the higher boiling point. compounds higher boiling point Xe Rn GeCl GeH, '4. Sil, SiCl,
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter14: Aldehydes And Ketones
Section: Chapter Questions
Problem 14.15E
Related questions
Question
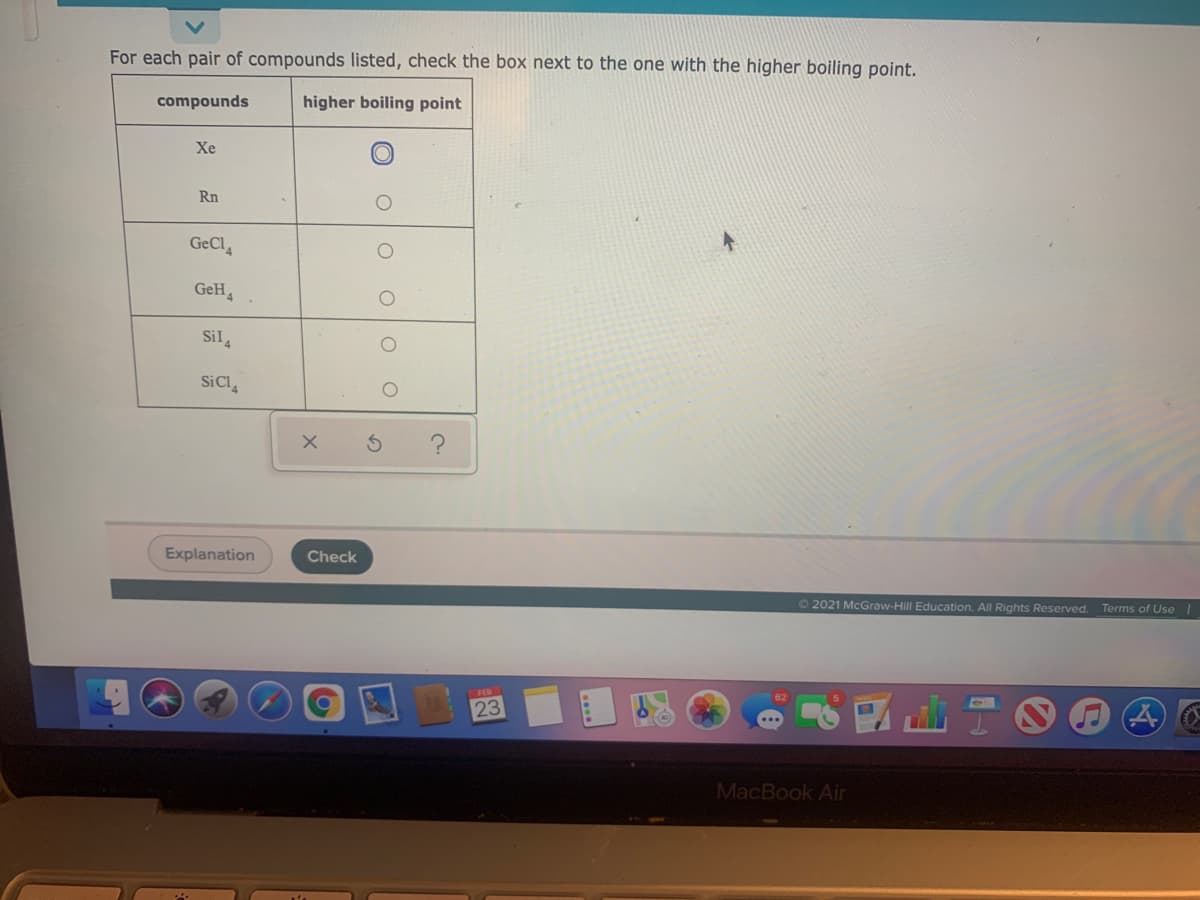
Transcribed Image Text:For each pair of compounds listed, check the box next to the one with the higher boiling point.
compounds
higher boiling point
Xe
Rn
GeCl
GeH,
Sil,
SiCl,
Explanation
Check
22021 McGraw-Hill Education. All Rights Reserved.
Terms of Use
23
MacBook Air
Expert Solution
Introduction
Boiling point of a molecule is the temperature at which its vapour pressure equals with the atmospheric pressure.
Boiling point increases with the molecular mass of the compound.
Type of intermolecular force also influence the boiling point.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning