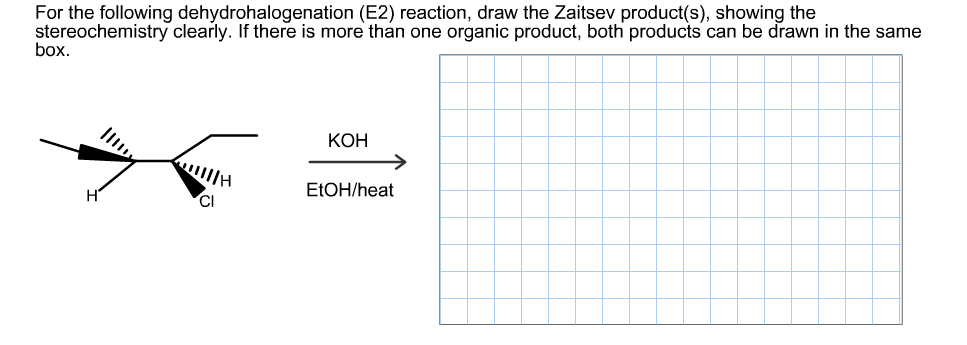
For the following dehydrohalogenation (E2) reaction, draw the Zaitsev product(s), showing the stereochemistry clearly. If there is more than one organic product, both products can be drawn in the same box КОН E1OH/heat H
Q: Rank the nucleophiles in each group in order of increasing nucleophilicity. а. "ОН, NH, Ha0 b. "OH,…
A: The reagents which have a greater affinity for the positively charged species are known as…
Q: Consider the attached E2 reaction Draw the by-products of the reaction and use curved arrows to…
A: Given:
Q: Prepare each compound from cyclopentanol. More than one step may be needed.llustrate the mechanism…
A:
Q: Draw and name the major product of the following E2 reaction. Clearly show any stereochemical…
A:
Q: the product(s) reaction below. cách product reaction mechanism that produced it (SN2/SN1/E1/E2) and…
A: Stereochemistry only change in SN2 Reaction because of the fact that Backside attack of…
Q: Consider the E2 elimination of 3-bromopentane with hydroxide. :Br CH3 product + Br+ H2O H CH3CH2…
A: E2 mechanism is a bimolecular elimination method. It is also known as concerted mechanism because…
Q: What is the main product of the E2-elimination reaction shown in the box? Br C,Hg C-C-CHs H CH3…
A: Given : Reaction of Structure Containing Bromine group with CH3CH2ONa To find : Product by E2 -…
Q: HC=CH The synthesis above can be performed with some combination of the reagents listed below. Give…
A: For deprotonation of acetylene we need strong base such as NaNH2.
Q: CH3 + NaOH an alkene + NaCl + H,0
A:
Q: Propose a mechanism for the following transformations, use curly arrows to show electron movement…
A: Small base favors formation of greater substituted product Bulky base favors less substituted…
Q: a good leaving group such as iodide. .CO₂H base НО 15 17 co Mecarmis SN2 ميكا تكيه ها التفاعل + TM3…
A:
Q: Draw the major organic product of the reaction. Indicate the stereochemistry via wedge-and-dash…
A: Given equation is example of a famous reaction, williamson ether synthesis.This an SN2…
Q: Draw the major E2 elimination product from the following alkyl halide. CH(CH2 HO CH, a draw…
A:
Q: Draw an alkyl bromide with proper stereochemistry that can be used to synthesize the given alkene as…
A: Answer: To form the desired alkene we need a strong non-bulky base that attacks on alkyl bromide…
Q: The molecule, 2-bromo-1,3-diethyl-1,4-dimethylcyclohexane undergoes an SN1/E1 reaction in the…
A: i. In the first step the leaving group Br departs to give carbocation. ii. Due to migration of most…
Q: Consider the following two reactions. Which is more highly regioselective, and why? Reaction 1 `Cl +…
A: Regioselectivity arises when there is a possibility of formation of more than one product. Among all…
Q: Draw the product of the following reaction sequence, including stereochemistry. CH3 [1] BH3 [2]…
A: The given reactant is 1-methyl cyclohexene. The product formed from the given reaction is given…
Q: 1. Consider the dehydration of the following alcohols using a Stre a circle around the alcohols that…
A: Dehydration is an elimination reaction. 10 alcohols follow E2 mechanism. 20 alcohols follow both E2…
Q: Which product shown is the correct product formed in this multi-step reaction? Acetic Acid Chloride…
A:
Q: Draw the structure of the product that is formed when the compound shown below is treated with cold,…
A: When alkene on treatment with cold dilute basic KMnO4, it produce 1,2-Diols.
Q: Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any…
A:
Q: CI КОН ELOH H Нeat
A:
Q: он The transformation above can be performed with some reagent or combination of the reagents listed…
A:
Q: Which molecule would be most susceptible to re-arrangement during an E1 attack, as the electrophile?…
A: Ans: 2-Bromo-3-methylhexane involves rearrangement. See below for mechanism.
Q: Draw the major organic product of the reaction shown below. NazCr207 H2SO4, H20 • You do not have to…
A: The detailed solution of your question given below-- Since, we know that the alcohol oxidised into…
Q: Br CH3SH CH3SH h. Ph
A: In the questions ( H and M ), we will give Reaction mechanism and Final Product(s). You can see…
Q: Predict the stereochemical outcome for the following E2 reaction. Draw only the major product of the…
A: Given reaction: We have to find the major product of the reaction.
Q: Draw the main organic products of the reaction. Indicate the stereochemistry, including all hydrogen…
A:
Q: Draw the product of the reaction shown below. Use wedge and dash bonds to indicate relative…
A: Given reaction is cis dihydroxylation reaction.
Q: Which synthetic method below correctly does the following conversion? СНО СНО Br A) (1) Mg, diethyl…
A: When aldehdye undergoes a reaction with alcohol, acetal is produced. When alkyl halide undergoes a…
Q: Which of the following reagent reacts with an alkene that will yield an anti- or non-Markovnikov…
A: Given statement is : Which of the following reagent reacts with an alkene that will yield an anti-…
Q: Using curved-arrow notation, draw out the mechanism for the following reaction. Be sure to include…
A: Alkenes are electron-rich species. They easily undergo an addition reaction in the presence of Br2.…
Q: Rank the alkyl halides in each group in order of increasing reactivity in an E2 reaction.
A: E2 type of reaction is second order elimination reaction in which the rate of reaction depends on…
Q: Which of the following statement is correct? A. E2 is a concerted reaction in which bonds break and…
A: The statements given are,
Q: Draw the products of the two step reaction sequence shown below. Use wedge and dash bonds to…
A: Here we are required to find the major product of the reaction .
Q: Complete the following reactions by providing the missing product(s). Determine what mechanism…
A:
Q: Draw the major organic product(s) of the following reaction. DMF Br + NaCN • You do not have to…
A: As the given halide is primary halide so the reaction occurred via SN2 mechanism.
Q: For each of the following reactions, using one or more "-R", you may abbreviate portions of the…
A: Since you have asked multiple question, we will solve the first question for you. If youwant any…
Q: What alkenes are formed from attached alkyl halide by an E1 reaction? Use the Zaitsev rule to…
A: In an E1 elimination reaction, the more substituted alkene is formed as the major product.
Q: The transformation above can be performed with some reagent or combination of the reagents listed…
A: The above given transformation (convention) of one molecule into another molecule can be carried out…
Q: Draw the products of the two step reaction sequence shown below. Use wedge and dash bonds to…
A: The reaction proceeds via SN2 path way. In the first step the lone pair electrons on O atom attack…
Q: 4. Draw the appropriate Newman projection (you can just draw the 1 that matters when you determine…
A:
Q: H. The transformations above can be performed with some combination of the reagents listed below.…
A: Organic reactions are those in which organic reactant react to form organic products.
Q: 1. Draw skeletal structures for the missing reagent or predict the product of the foliowing…
A: Skeletal structure to the missing reagant and the product of the following electrophilic addition…
Q: In each reaction box, place the best reagent and conditions from the list below
A: In the given transformation, cyclohexanone is being converted into 3-ethyl cyclohexanone.
Q: Complete the following reactions by providing the missing product(s). Determine what mechanism…
A:
Q: What is the main product of the E2-elimination reaction shown in the box? CH;CH,ONa ? Br CH;CH,OH A)…
A: Elimination reactions are those organic reactions one or more atoms are removed from the molecule in…
Q: 5. E2 elimination of the substrate below with potassium hydroxide affords a single diastereomer of…
A: When this type of alkyl halide is treated with base then elimination reaction takes place . By…
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

- For the dehydrohalogenation (E2) reaction, draw the Zaitsev product, showing the stereochemistry clearly. You might find it helpful to make a model of the starting material to determine the correct conformation.Rank the following E2 reactions in order of increasing rate. Show the final product.For the dehydrohalogenation (E2) reaction shown, draw the major organic product, including stereochemistry.
- In the box to the left of each reaction below, write the mechanism by which it occurs (could be SN1, SN2, or E1, or even 2 of them). Then draw the product(s).1. Draw a reasonable arrow-pushing mechanism for the transformation shown along. 2. Identify nucleophiles and electrophiles 3. Name any type of reactions taking place like E2 or E1 4. Account for any regio- or stereoselectivityGiven that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5 −is a phenyl group, a benzene ring bonded to another group.
- Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5– is a phenyl group, a benzene ring bonded to another group.Draw the major organic product of this E1 elimination reaction. Ignore byproducts.a. What are the products of following reactions?b. Write the reaction mechanism for each using the right arrows and define it as Sn2, Sn1, E2 or E1.c. Explain why do you choose that product and mechanism or in case something else happens explain why.

