For the following molecules: a. Draw the dot structure (please draw out all of the shells) b. Place a circle around the electrons being shared C. Indicate if the molecules is polar or non-polar. If the molecule is polar, put in the partial charges HCl(g) (one atom of hydrogen and one atom of chlorine) NH3 (one atom of nitrogen and three atoms of hydrogen) Covalent bonds (continued) H2S (1 Sulfur atom and 3 hydrogen) PH3 (1 phosphorus and 3 hydrogens)
For the following molecules: a. Draw the dot structure (please draw out all of the shells) b. Place a circle around the electrons being shared C. Indicate if the molecules is polar or non-polar. If the molecule is polar, put in the partial charges HCl(g) (one atom of hydrogen and one atom of chlorine) NH3 (one atom of nitrogen and three atoms of hydrogen) Covalent bonds (continued) H2S (1 Sulfur atom and 3 hydrogen) PH3 (1 phosphorus and 3 hydrogens)
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 22STP
Related questions
Question
How do you solve this question ?

Transcribed Image Text:For the following molecules:
a.
Draw the dot structure (please draw out all of the shells)
b.
Place a circle around the electrons being shared
C.
Indicate if the molecules is polar or non-polar.
If the molecule is polar, put in the partial charges
HCl(g) (one atom of hydrogen and one atom of chlorine)
NH3 (one atom of nitrogen and three atoms of hydrogen)

Transcribed Image Text:Covalent bonds (continued)
H2S (1 Sulfur atom and 3 hydrogen)
PH3 (1 phosphorus and 3 hydrogens)
Expert Solution
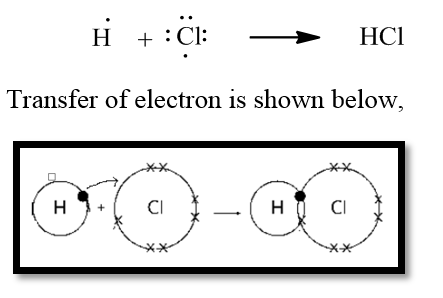
The dot structure of HCl (hydrogen + chlorine) is given below,
Hi, since there are multiple questions posted, we will answer first question. If you want any specific question to be answered then please submit that question only or specify the question number in your message.
Thank you.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning