For the following reaction, 12.9 grams of chlorine gas are allowed to react with 57.6 grams of sodium iodide chlorine(g) sodium iodide(s) sodium chloride(s)+ iodine(s) What is the maximum amount of sodium chloride that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams
For the following reaction, 12.9 grams of chlorine gas are allowed to react with 57.6 grams of sodium iodide chlorine(g) sodium iodide(s) sodium chloride(s)+ iodine(s) What is the maximum amount of sodium chloride that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 145AE: A potential fuel for rockets is a combination of B5H9 and 0 2. The two react according to the...
Related questions
Question
can you please answer the question exactly how they want it up there.
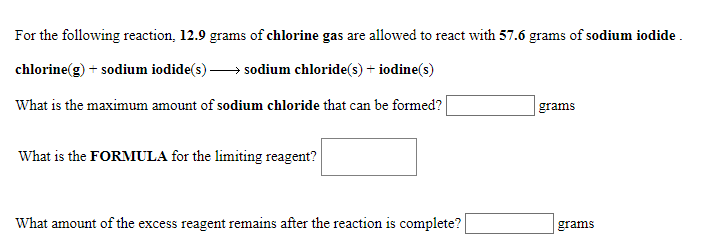
Transcribed Image Text:For the following reaction, 12.9 grams of chlorine gas are allowed to react with 57.6 grams of sodium iodide
chlorine(g)
sodium iodide(s)
sodium chloride(s)+ iodine(s)
What is the maximum amount of sodium chloride that can be formed?
grams
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?
grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning