For the following reaction, draw the major organic product and select the best name for the organic reactant. If there is more than one major product, both may be drawn in the same box. (Indicate stereochemistry with the wedge and dash.) When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect HBr На Select the best name for the organic reactant: (Z)-3,4-dimethyl-4-pentene (E)-2,3-dimethyl-1-pentene (Z)-3,4-dimethyl-4-hexene (E)-3,4-dimethyl-2-hexene
For the following reaction, draw the major organic product and select the best name for the organic reactant. If there is more than one major product, both may be drawn in the same box. (Indicate stereochemistry with the wedge and dash.) When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect HBr На Select the best name for the organic reactant: (Z)-3,4-dimethyl-4-pentene (E)-2,3-dimethyl-1-pentene (Z)-3,4-dimethyl-4-hexene (E)-3,4-dimethyl-2-hexene
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 25CTQ
Related questions
Question
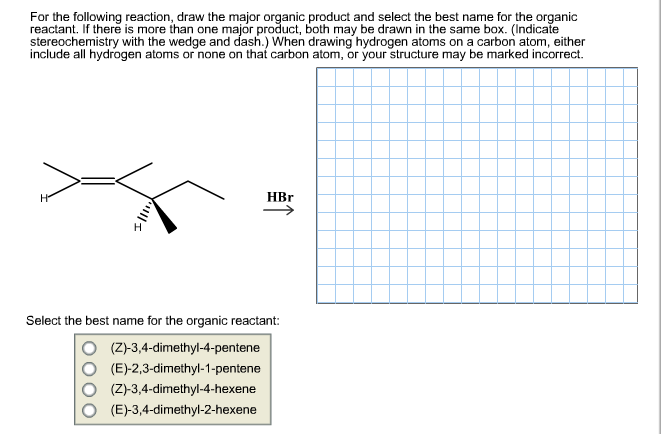
Transcribed Image Text:For the following reaction, draw the major organic product and select the best name for the organic
reactant. If there is more than one major product, both may be drawn in the same box. (Indicate
stereochemistry with the wedge and dash.) When drawing hydrogen atoms on a carbon atom, either
include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect
HBr
На
Select the best name for the organic reactant:
(Z)-3,4-dimethyl-4-pentene
(E)-2,3-dimethyl-1-pentene
(Z)-3,4-dimethyl-4-hexene
(E)-3,4-dimethyl-2-hexene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning