form a simple cubic unit cell 1. The anions of CsCl fill the octahedral holes 2. The anions NaCl and ZnS form a body-centered cubic unit cell 3. The cations of CsCI fill the tetrahedral holes 4. The cations of NaCl fill the cubic holes form a face-centered cubic unit cell 5. The cations of ZnS fill every other tetrahedral hole
form a simple cubic unit cell 1. The anions of CsCl fill the octahedral holes 2. The anions NaCl and ZnS form a body-centered cubic unit cell 3. The cations of CsCI fill the tetrahedral holes 4. The cations of NaCl fill the cubic holes form a face-centered cubic unit cell 5. The cations of ZnS fill every other tetrahedral hole
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section9.6: Crystalline Solids
Problem 9.13E
Related questions
Question
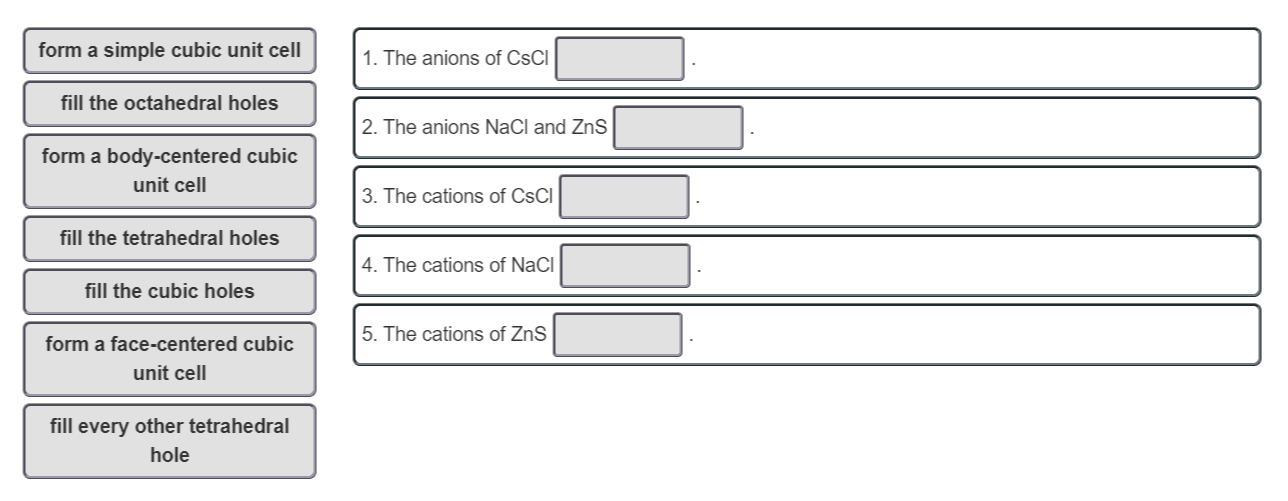
Transcribed Image Text:form a simple cubic unit cell
1. The anions of CsCl
fill the octahedral holes
2. The anions NaCl and ZnS
form a body-centered cubic
unit cell
3. The cations of CsCI
fill the tetrahedral holes
4. The cations of NaCl
fill the cubic holes
form a face-centered cubic
unit cell
5. The cations of ZnS
fill every other tetrahedral
hole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
