Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water (choose one) (choose one) 500. mL of pure water (choose one) (choose one) ? X Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Lenovo Esc + Home Insert Dele End FnLock F2 F1 F3 F4 F5 F6 F8 F7 F9 F10 F11 F12 & Bac 4 2 3 6 7 Q T Y R P +II LO S LWJ Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water (choose one) (choose one) 500. mL of pure water (choose one) (choose one) ? X Explanation Check Lenovo
Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water (choose one) (choose one) 500. mL of pure water (choose one) (choose one) ? X Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Lenovo Esc + Home Insert Dele End FnLock F2 F1 F3 F4 F5 F6 F8 F7 F9 F10 F11 F12 & Bac 4 2 3 6 7 Q T Y R P +II LO S LWJ Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water (choose one) (choose one) 6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water (choose one) (choose one) 500. mL of pure water (choose one) (choose one) ? X Explanation Check Lenovo
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 17E
Related questions
Question
For liquids are described in the table below use the second column of the table to explain the order out there freezing points in the third column to explain the order of the appointment points.
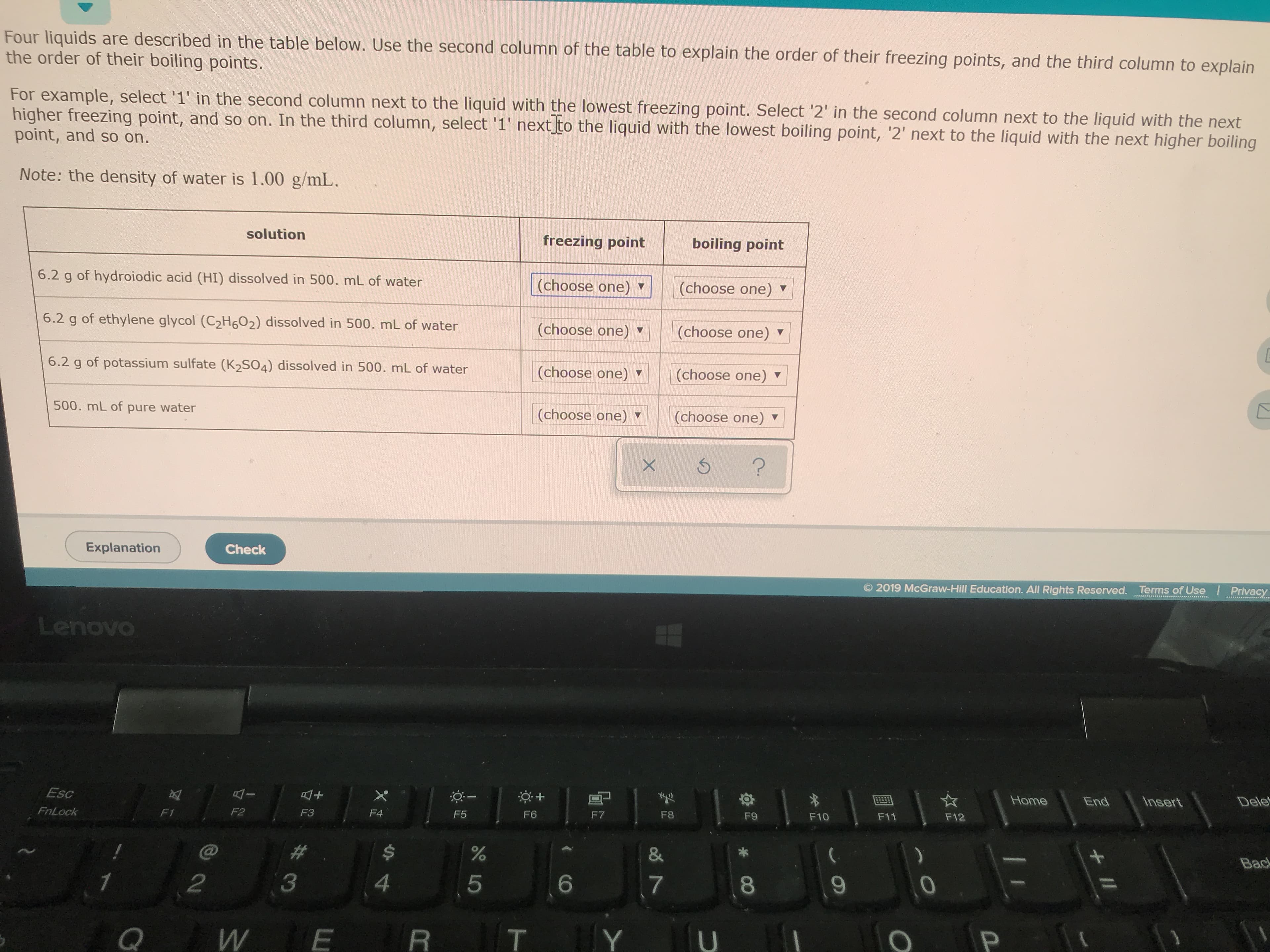
Transcribed Image Text:Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain
the order of their boiling points.
For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next
higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling
point, and so on.
Note: the density of water is 1.00 g/mL.
solution
freezing point
boiling point
6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water
(choose one)
(choose one)
6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water
(choose one)
(choose one)
6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water
(choose one)
(choose one)
500. mL of pure water
(choose one)
(choose one)
?
X
Explanation
Check
2019 McGraw-Hill Education. All Rights Reserved.
Terms of Use Privacy
Lenovo
Esc
+
Home
Insert
Dele
End
FnLock
F2
F1
F3
F4
F5
F6
F8
F7
F9
F10
F11
F12
&
Bac
4
2
3
6
7
Q
T
Y
R
P
+II
LO
S
LWJ
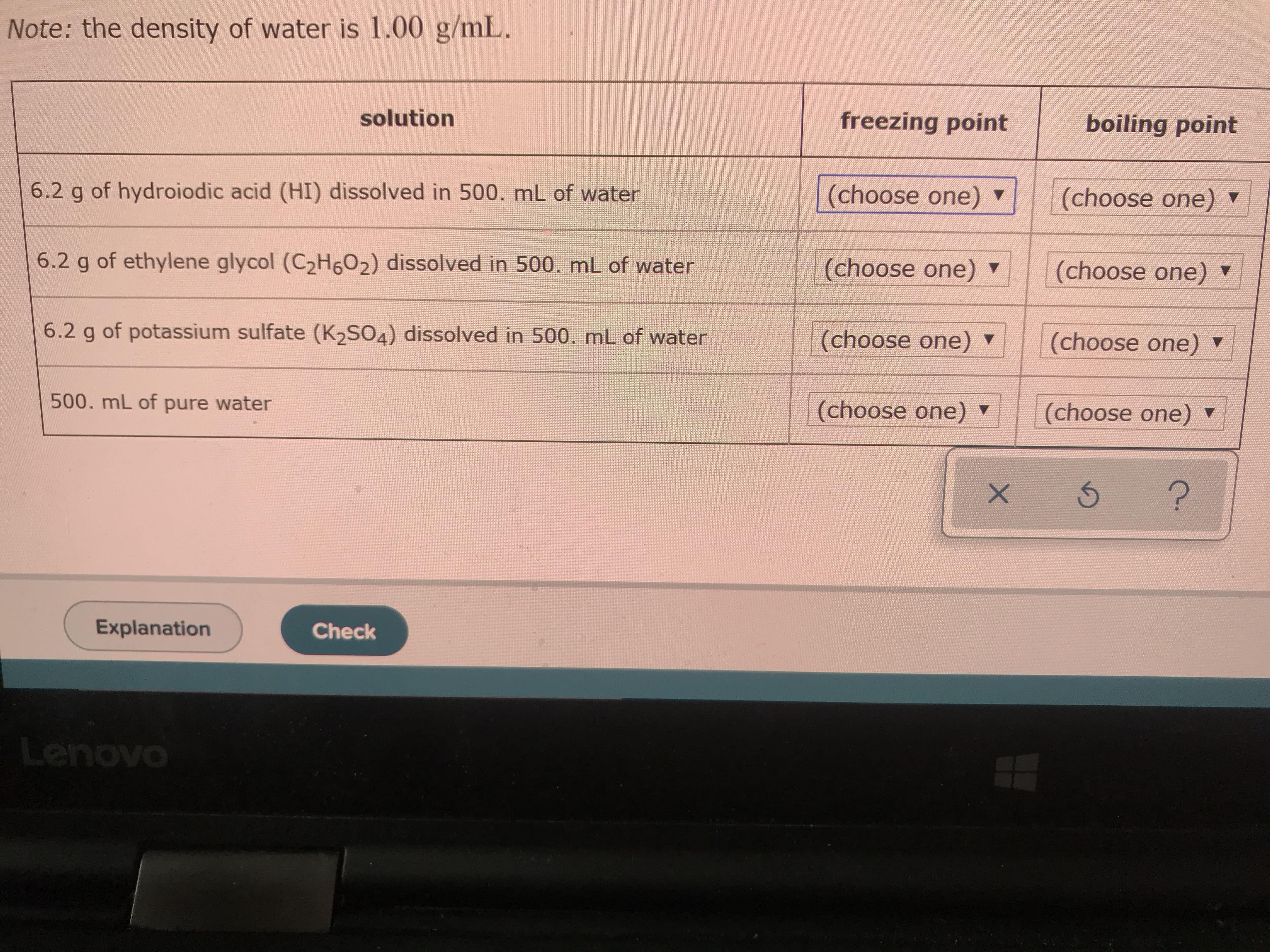
Transcribed Image Text:Note: the density of water is 1.00 g/mL.
solution
freezing point
boiling point
6.2 g of hydroiodic acid (HI) dissolved in 500. mL of water
(choose one)
(choose one)
6.2 g of ethylene glycol (C2H602) dissolved in 500. mL of water
(choose one)
(choose one)
6.2 g of potassium sulfate (K2SO4) dissolved in 500. mL of water
(choose one)
(choose one)
500. mL of pure water
(choose one)
(choose one)
?
X
Explanation
Check
Lenovo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning