Gaseous butane (CHa CH2)CH3 will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H20). Suppose 49. g of butane is mixed with 150. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g x10 ? Submit Assignment Continue 2019 McGrawH Eucation All Rights Reserved Terms ofUse 1 Privacy MacBook Pro F11 F10 F9 F8 000 F 7 F6 F5 F 4 F3 F2 & X
Gaseous butane (CHa CH2)CH3 will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H20). Suppose 49. g of butane is mixed with 150. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g x10 ? Submit Assignment Continue 2019 McGrawH Eucation All Rights Reserved Terms ofUse 1 Privacy MacBook Pro F11 F10 F9 F8 000 F 7 F6 F5 F 4 F3 F2 & X
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 101AE: This year, like many past years, you begin to feel very sleepy alter eating a large helping of...
Related questions
Question
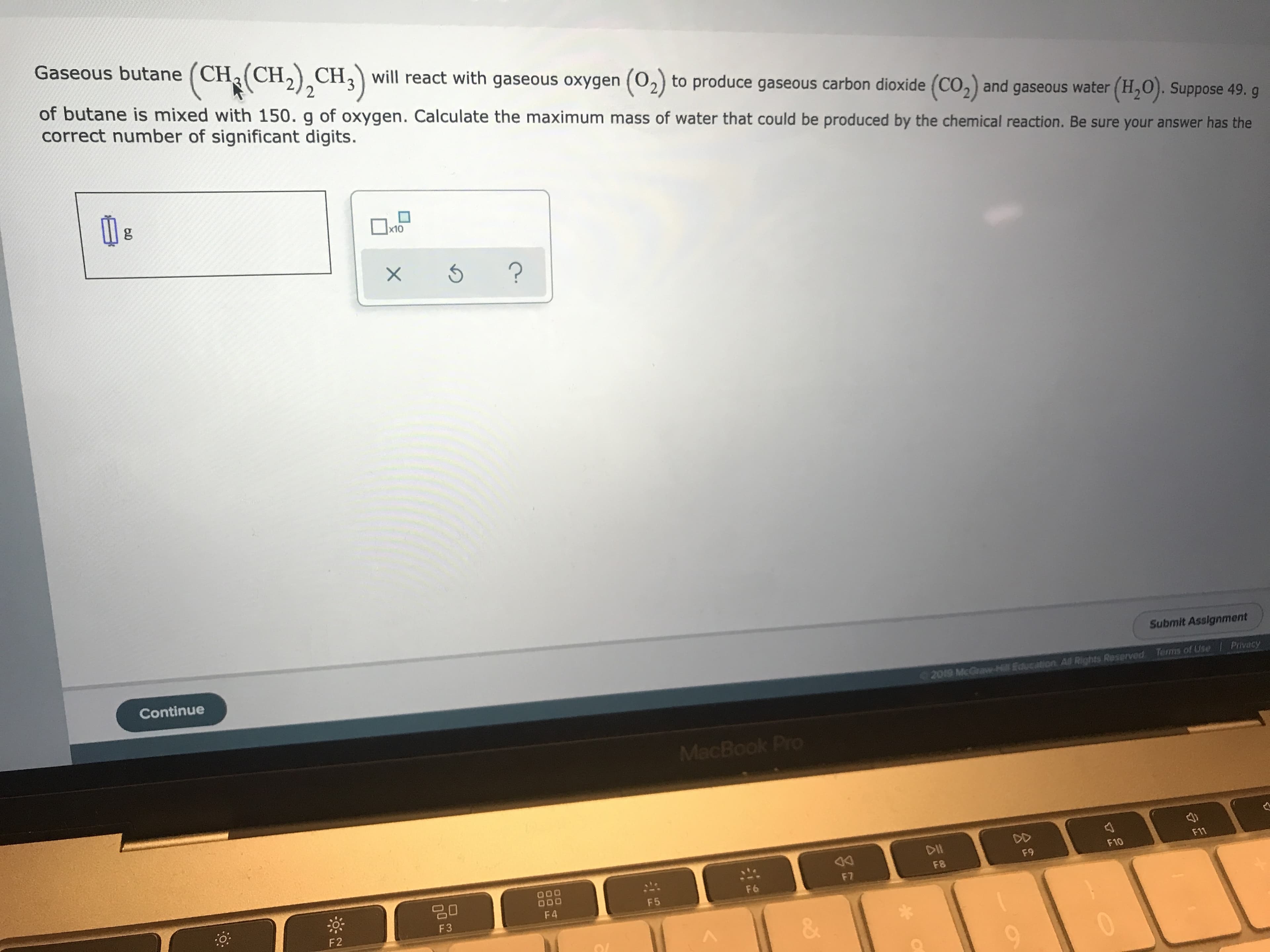
Transcribed Image Text:Gaseous butane (CHa CH2)CH3
will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H20). Suppose 49. g
of butane is mixed with 150. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the
correct number of significant digits.
g
x10
?
Submit Assignment
Continue
2019 McGrawH Eucation All Rights Reserved Terms ofUse 1 Privacy
MacBook Pro
F11
F10
F9
F8
000
F 7
F6
F5
F 4
F3
F2
&
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning