Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane. Select each of the reactions and observe the reactants taking part in the reactions. Suppose you are carrying out each of these reactions starting with five moles of each reactant. Observe that some reactants are in excess whereas some reactants limit the amount of products formed. Classify each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant. Drag the appropriate items to their respective bins. ► View Available Hint(s) O₂ in combustion of methane Limiting reagent N₂ in formation ammonia H₂ in formation of ammonia H₂ in formation of water O₂ in formation of water Excess reagent CH4 in combustion of methane Reset Help
Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane. Select each of the reactions and observe the reactants taking part in the reactions. Suppose you are carrying out each of these reactions starting with five moles of each reactant. Observe that some reactants are in excess whereas some reactants limit the amount of products formed. Classify each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant. Drag the appropriate items to their respective bins. ► View Available Hint(s) O₂ in combustion of methane Limiting reagent N₂ in formation ammonia H₂ in formation of ammonia H₂ in formation of water O₂ in formation of water Excess reagent CH4 in combustion of methane Reset Help
Chapter3: Using Spreadsheets In Analytical Chemistry
Section: Chapter Questions
Problem 3.2QAP
Related questions
Question

Transcribed Image Text:In the Sandwiches mode, select the "Cheese" option and observe the equation given for the preparation of a cheese sandwich.
In the equation, set the number of bread slices to "2" and the number of cheese slices to "1." You will see that the product formed by these three ingredients is one cheese sandwich.
Suppose you have 38 bread slices and 28 cheese slices. How many cheese sandwiches can you make?
Express your answer as an integer.
► View Available Hint(s)
VΠΙ ΑΣΦ
?
sandwiches
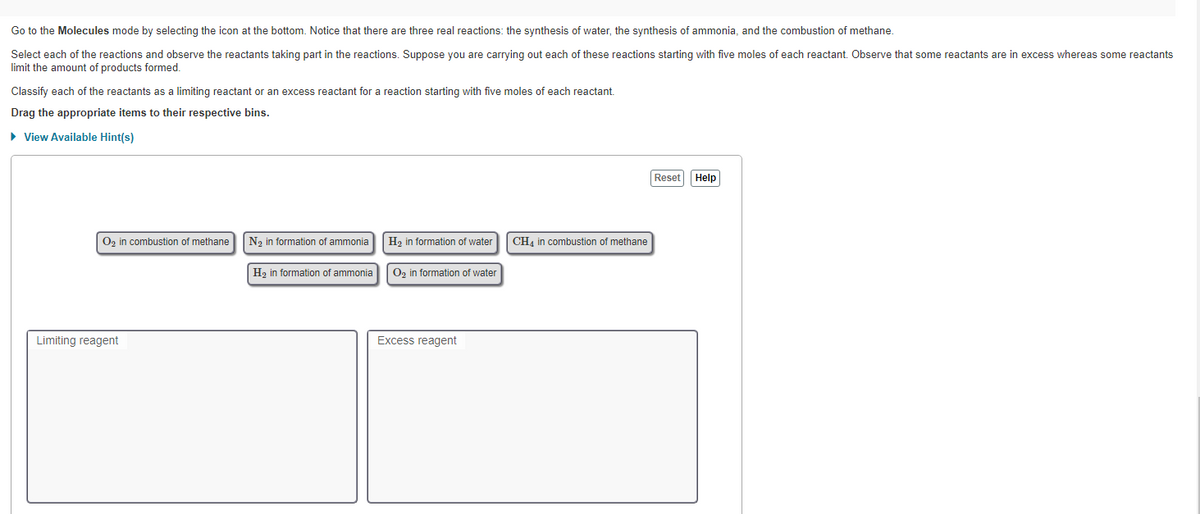
Transcribed Image Text:Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane.
Select each of the reactions and observe the reactants taking part in the reactions. Suppose you are carrying out each of these reactions starting with five moles of each reactant. Observe that some reactants are in excess whereas some reactants
limit the amount of products formed.
Classify each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant.
Drag the appropriate items to their respective bins.
► View Available Hint(s)
O₂ in combustion of methane
Limiting reagent
N₂ in formation of ammonia
H₂ in formation of ammonia
H₂ in formation of water
O₂ in formation of water
Excess reagent
CH4 in combustion of methane
Reset
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning