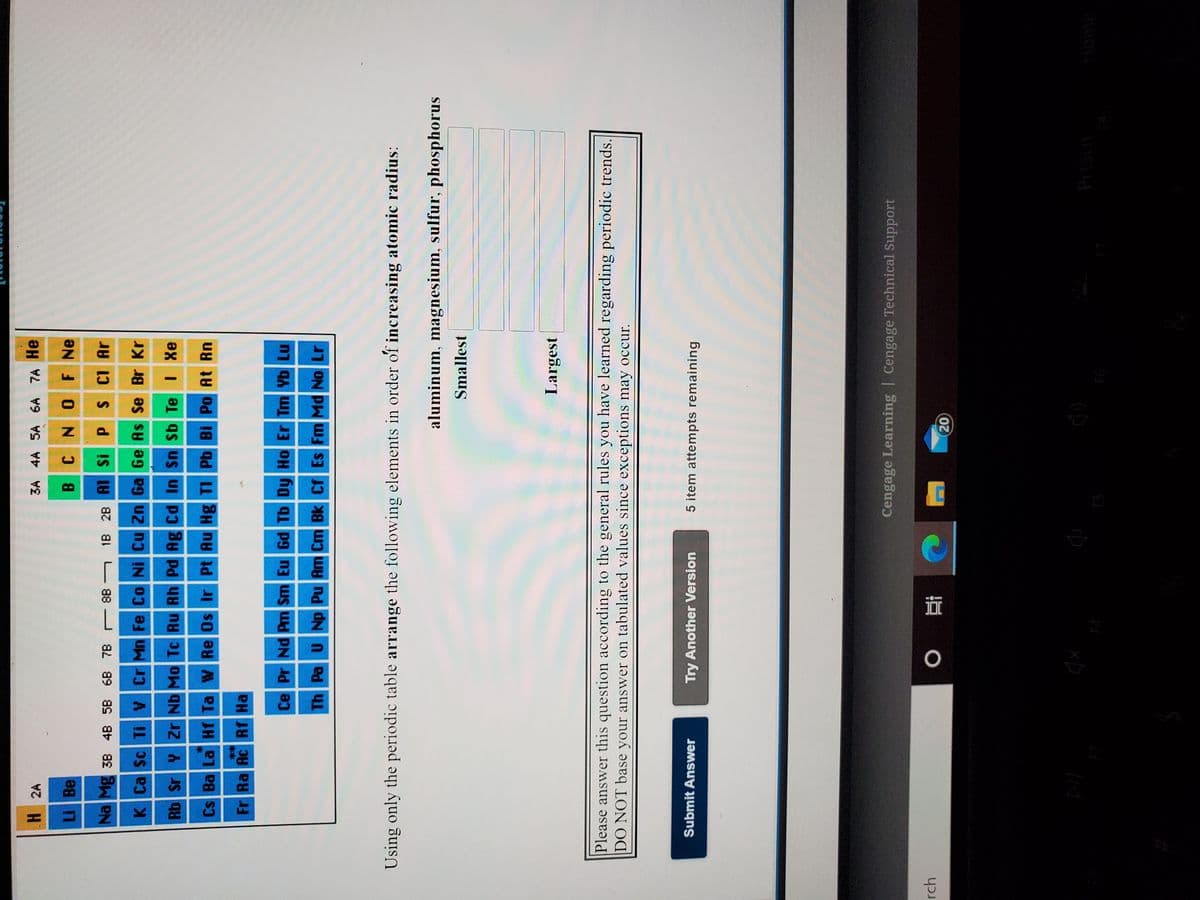
H 2A 3A 4A SA 6A 7A He LI Be ƏN 1 0N 3 8 Na Mg 3B 4B 58 6B 7B r 8B - 18 28 AI Si PS CI Ar K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y ZrNb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr Using only the periodic table arrange the following elements in order of increasing atomic radius: aluminum, magnesium, sulfur, phosphorus Smallest Largest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur.
H 2A 3A 4A SA 6A 7A He LI Be ƏN 1 0N 3 8 Na Mg 3B 4B 58 6B 7B r 8B - 18 28 AI Si PS CI Ar K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y ZrNb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr Using only the periodic table arrange the following elements in order of increasing atomic radius: aluminum, magnesium, sulfur, phosphorus Smallest Largest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur.
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 43E: Early tables of atomic weights (masses) were generated by measuring the mass of a substance that...
Related questions
Question
100%
Transcribed Image Text:20
H 2A
3A 4A 5A 6A 7A He
Li Be
CNOF Ne
B.
Na Mg 3B 4B 5B 6B 7B 8B 1B 2B Al Si P
S CI Ar
K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
**
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr
Using only the periodic table arrange the following elements in order of increasing atomic radius:
aluminum, magnesium, sulfur, phosphorus
Smallest
Largest
Please answer this question according to the general rules you have learned regarding periodic trends.
DO NOT base your answer on tabulated values since exceptions may occur.
Submit Answer
Try Another Version
5 item attempts remaining
Cengage Learning | Cengage Technical Support
rch
近。
PrtSen
93
Expert Solution
Step 1
In period as moving from left to right, atomic radius decreases due to decrease in nuclear charge.
Aluminum, Magnesium , sulfur, phosphorus belongs to 3rd period of periodic table.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning