H20 NO2+ PO NO3 Crude Sketch Calculations (# of valence electrons, # of bonds, etc. Lewis Structure # electron groups, electron group geometry # of bonded atoms, molecular shane DFocus
H20 NO2+ PO NO3 Crude Sketch Calculations (# of valence electrons, # of bonds, etc. Lewis Structure # electron groups, electron group geometry # of bonded atoms, molecular shane DFocus
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.97PAE: 7.97 Consider the structure shown below for as well as any other important resonance structures. (a)...
Related questions
Question
Just need help with the last two 3,4
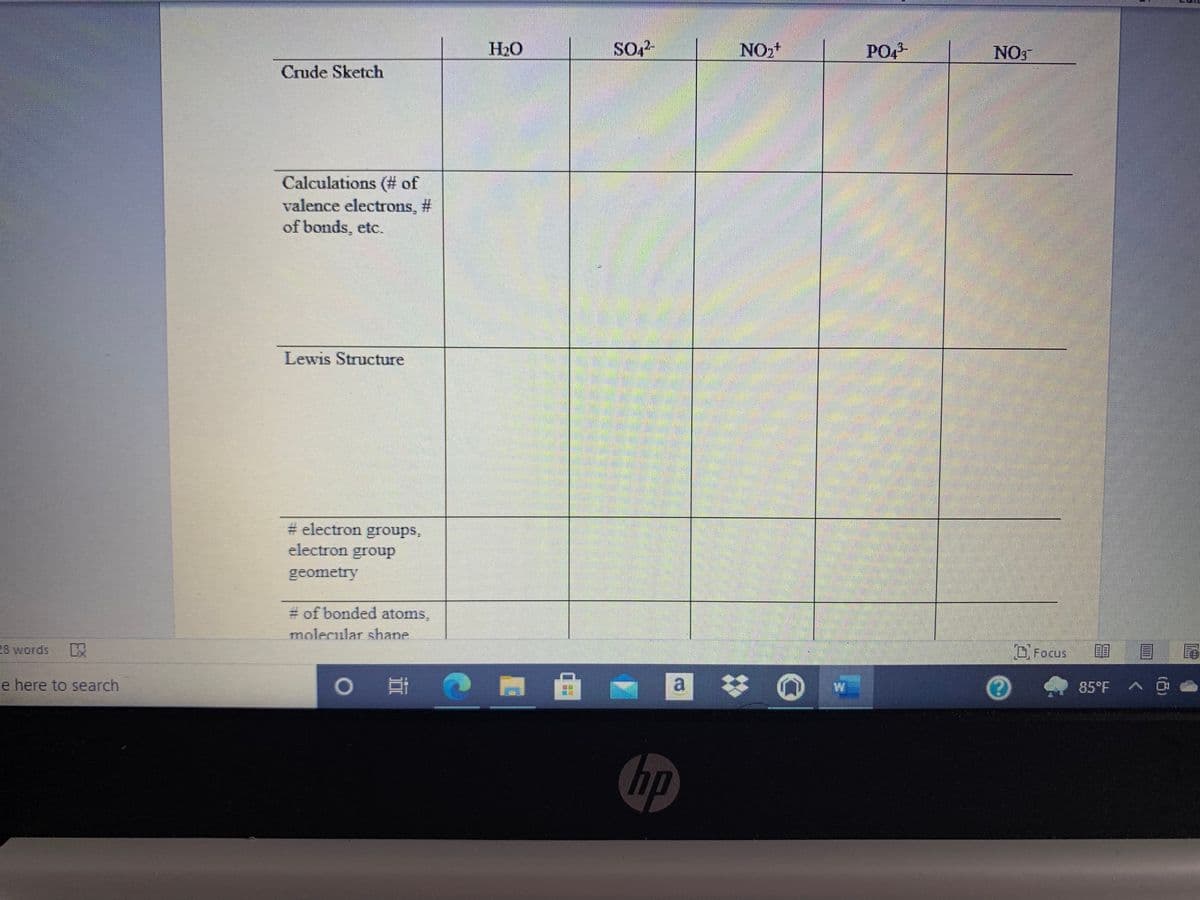
Transcribed Image Text:H20
NO,+
PO,3-
NO
Crude Sketch
Calculations (# of
valence electrons, #
of bonds, etc.
Lewis Structure
# electron groups,
electron group
geometry
# of bonded atoms,
molecular shane
28 words
OFocus
e here to search
a *
85°F A O
hp
Transcribed Image Text:FHeading 4
A Select
Font
Paragraph
Styles
Editing
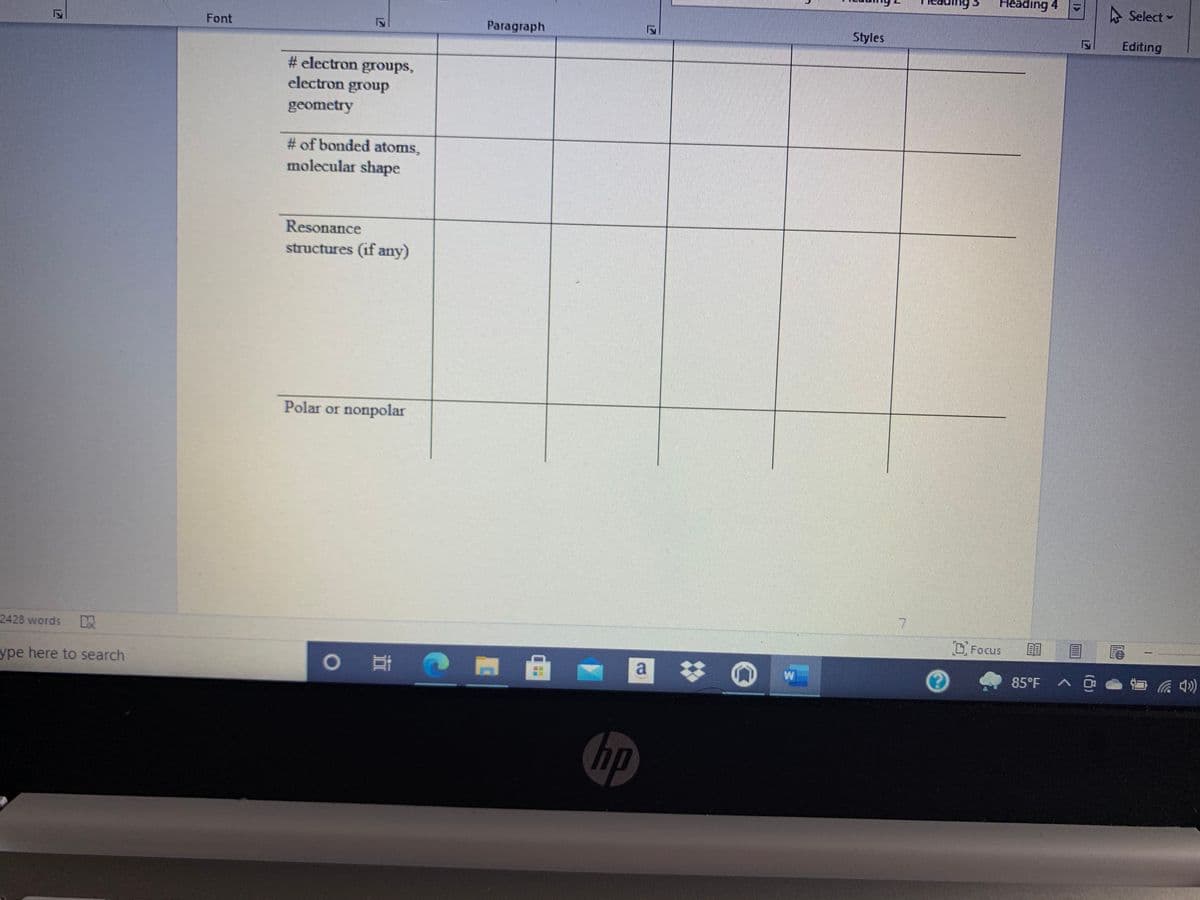
# electron groups,
electron group
geometry
# of bonded atoms,
molecular shape
Resonance
structures (if any)
Polar or nonpolar
7.
OFocus
2428 words
o 耳
85°F A a
a
ype here to search
hp
12
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
