he clenty Mh have no ted from d ond c Calculatn Vulneit can 4. A group of students determine the density of a sample of liquid éthylene glycol. Each student uses a graduated cylinder to obtain the ethylene glycol volume and a balance to obtain the ethylene glycol mass. Their density values are shown in the table below. a) Determine the average density and the standard deviation in this density data set. You may use your graphic calculator or Excel. Density (g/mL) Density Student I.II (g/mL) 2 1.21 1.30 4 1.01 5 I.13 beaker graduated cylinder b) During the second part of the laboratory activity, the students decide to determine the density of ethylene glycol using a beaker. The results are using the graduated cylinder. Which glassware yielded the more precise measurements? Explain. displayed in the graph above, along with those obtained Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision) 14 XXXX X x x X X
he clenty Mh have no ted from d ond c Calculatn Vulneit can 4. A group of students determine the density of a sample of liquid éthylene glycol. Each student uses a graduated cylinder to obtain the ethylene glycol volume and a balance to obtain the ethylene glycol mass. Their density values are shown in the table below. a) Determine the average density and the standard deviation in this density data set. You may use your graphic calculator or Excel. Density (g/mL) Density Student I.II (g/mL) 2 1.21 1.30 4 1.01 5 I.13 beaker graduated cylinder b) During the second part of the laboratory activity, the students decide to determine the density of ethylene glycol using a beaker. The results are using the graduated cylinder. Which glassware yielded the more precise measurements? Explain. displayed in the graph above, along with those obtained Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision) 14 XXXX X x x X X
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.112E
Related questions
Question
Question 4 A and B
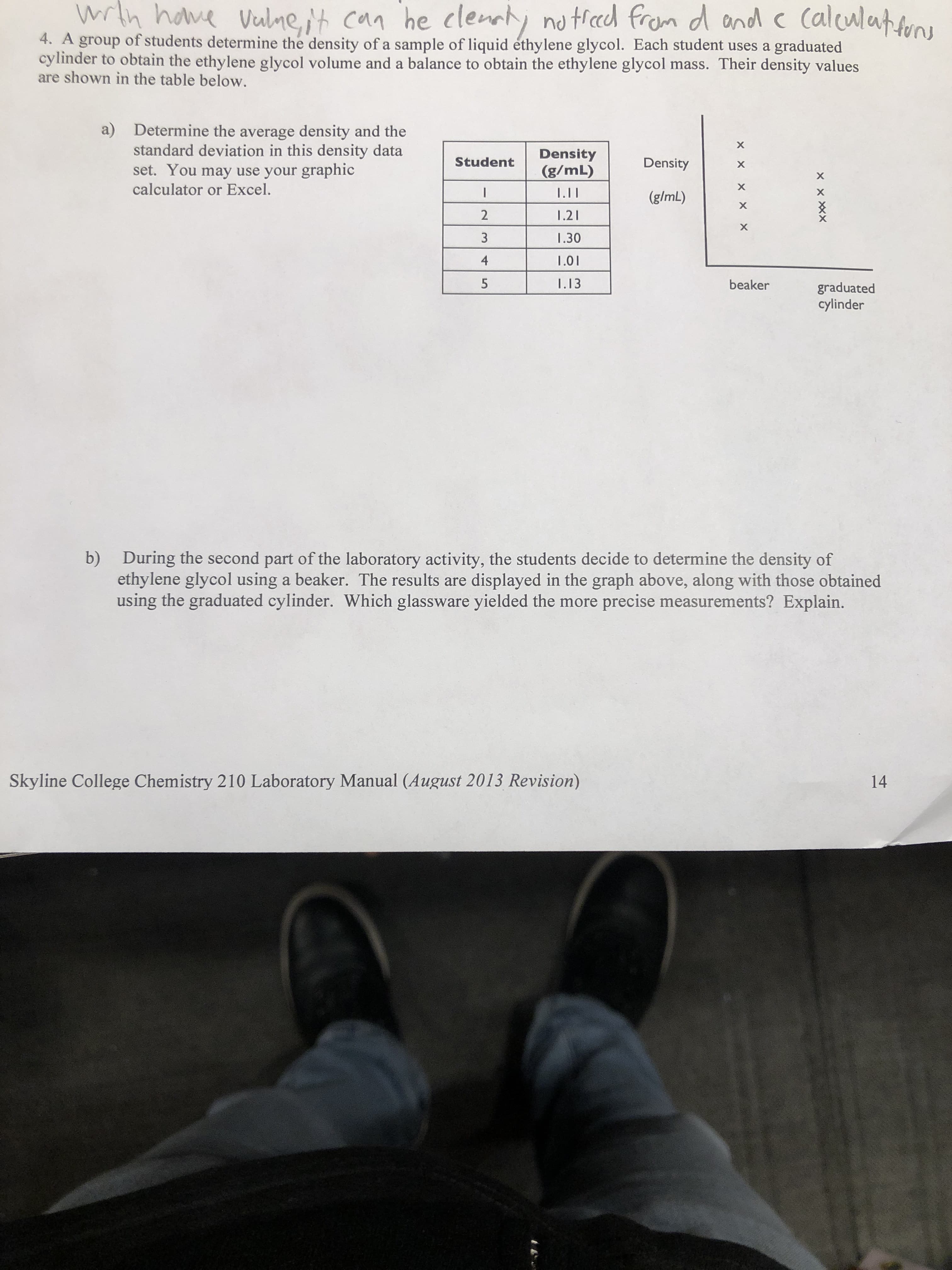
Transcribed Image Text:he clenty
Mh have
no ted from d ond c Calculatn
Vulneit can
4. A group of students determine the density of a sample of liquid éthylene glycol. Each student uses a graduated
cylinder to obtain the ethylene glycol volume and a balance to obtain the ethylene glycol mass. Their density values
are shown in the table below.
a)
Determine the average density and the
standard deviation in this density data
set. You may use your graphic
calculator or Excel.
Density
(g/mL)
Density
Student
I.II
(g/mL)
2
1.21
1.30
4
1.01
5
I.13
beaker
graduated
cylinder
b)
During the second part of the laboratory activity, the students decide to determine the density of
ethylene glycol using a beaker. The results are
using the graduated cylinder. Which glassware yielded the more precise measurements? Explain.
displayed in the graph above, along with those obtained
Skyline College Chemistry 210 Laboratory Manual (August 2013 Revision)
14
XXXX
X x x X X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning