Heat is added to a 9.0 kg piece of ice at a rate of 529.0 kW. How long will it take for the ice at 0.0° C to melt? (For water Lf= 334 kJ/kg and Ly = 2257 kJ/kg.) A) 0.0s B) 3000s C) 5.7 s D) 1,600,000 s
Heat is added to a 9.0 kg piece of ice at a rate of 529.0 kW. How long will it take for the ice at 0.0° C to melt? (For water Lf= 334 kJ/kg and Ly = 2257 kJ/kg.) A) 0.0s B) 3000s C) 5.7 s D) 1,600,000 s
Chapter5: Temperature And Heat
Section: Chapter Questions
Problem 13P: - (a) Compute the amount of heat needed to raise the temperature of 1 kg of water from its freezing...
Related questions
Question
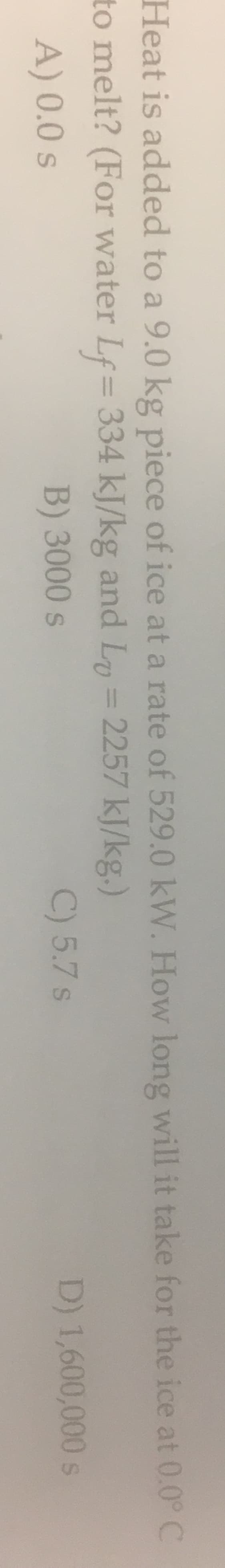
Transcribed Image Text:Heat is added to a 9.0 kg piece of ice at a rate of 529.0 kW. How long will it take for the ice at 0.0° C
to melt? (For water Lf= 334 kJ/kg and Ly = 2257 kJ/kg.)
A) 0.0s
B) 3000s
C) 5.7 s
D) 1,600,000 s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you



An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning



An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College