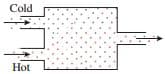
Helium at 200 kPa, 20 ∘C is heated by mixing it with argon at 200 kPa, 500 ∘C in an adiabatic chamber. Helium enters the chamber at 2 kg/s and argon at 0.6 kg/s. The mixture leaves at 200 kPa. (Figure 1) Part A Determine the temperature (T2) at the exit. Express your answer to four significant figures. Part B Determine the rate of entropy generation (S˙gen) due to mixing. Express your answer to three significant figures in kW/K.
Helium at 200 kPa, 20 ∘C is heated by mixing it with argon at 200 kPa, 500 ∘C in an adiabatic chamber. Helium enters the chamber at 2 kg/s and argon at 0.6 kg/s. The mixture leaves at 200 kPa. (Figure 1) Part A Determine the temperature (T2) at the exit. Express your answer to four significant figures. Part B Determine the rate of entropy generation (S˙gen) due to mixing. Express your answer to three significant figures in kW/K.
Welding: Principles and Applications (MindTap Course List)
8th Edition
ISBN:9781305494695
Author:Larry Jeffus
Publisher:Larry Jeffus
Chapter5: Shielded Metal Arc Welding Of Pipe
Section: Chapter Questions
Problem 12R: What is the purpose of a hot pass?
Related questions
Question
Helium at 200 kPa, 20 ∘C is heated by mixing it with argon at 200 kPa, 500 ∘C in an adiabatic chamber. Helium enters the chamber at 2 kg/s and argon at 0.6 kg/s. The mixture leaves at 200 kPa. (Figure 1)
Part A
Determine the temperature (T2) at the exit.
Express your answer to four significant figures.
Part B
Determine the rate of entropy generation (S˙gen) due to mixing.
Express your answer to three significant figures in kW/K.
Transcribed Image Text:Cold
Hot
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning