Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 18QAP
Related questions
Question
Which of the following is a strong acid in aqueous solution?
(a) Mg(OH)2
(b) NH3
(c) H2SO4
(d) CH3COOH
(e) AgCl
Expert Solution
Introduction
When an acid dissociates into its ions, then the ion formed is its conjugate base and when an base dissociates into its ions, then the ion formed is its conjugate acid. If the acid is strong, its conjugate base is weak or vice-versa. And if the base is strong, its conjugate acid is weak or vice-versa.
Explanation

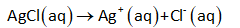
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning