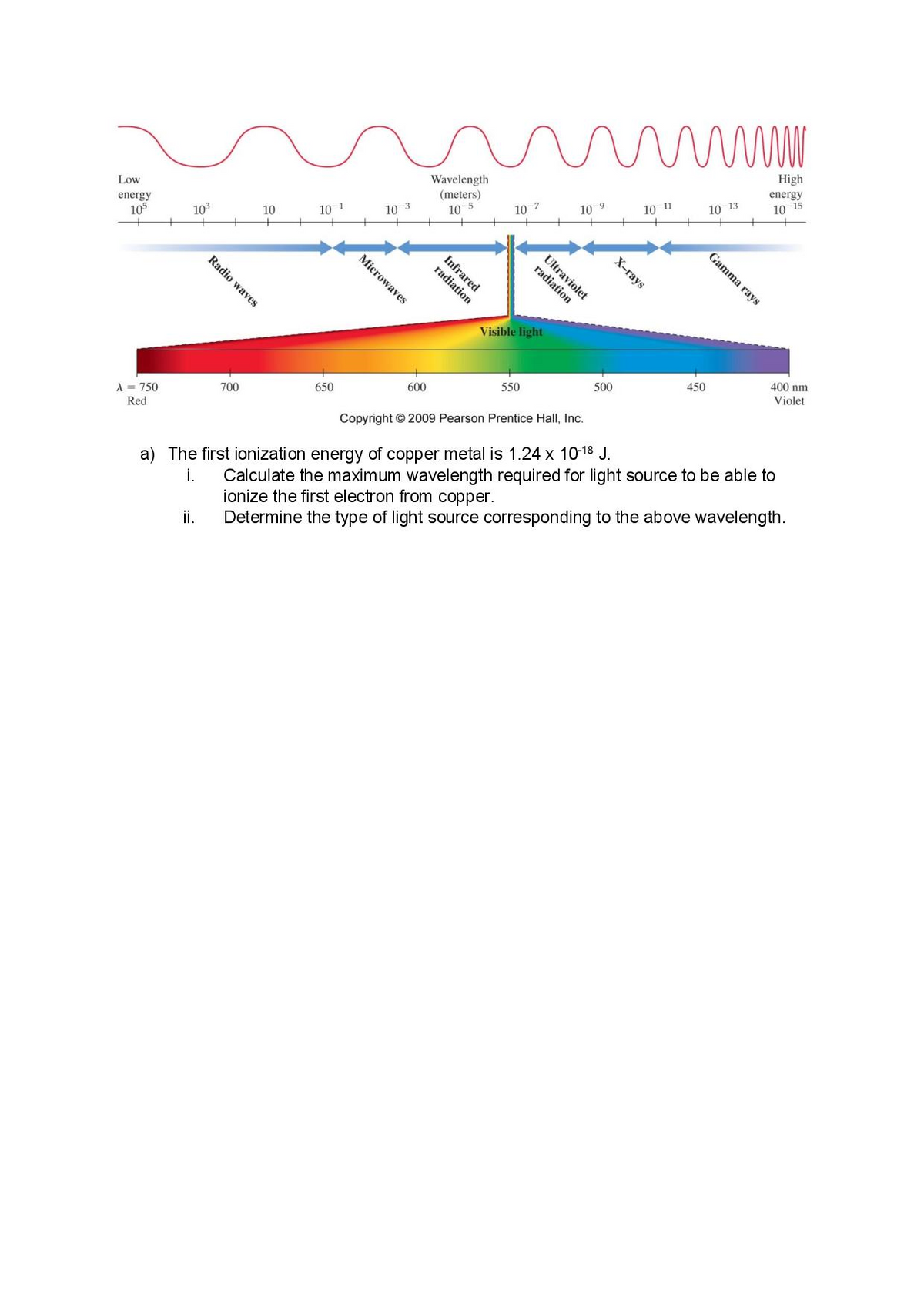
High energy 10-15 Wavelength (meters) 10-5 Low 10-11 10-13 10-7 10-9 energy 105 10 10-1 10-3 103 Ultraviolet X-rays Infrared radiation Visible light 400 nm 450 550 500 Violet 600 650 1 = 750 700 Red Copyright © 2009 Pearson Prentice Hall, Inc. a) The first ionization energy of copper metal is 1.24 x 10-18 J. i. ionize the first electron from copper. Determine the type of light source corresponding to the above wavelength. Calculate the maximum wavelength required for light source to be able to i. Gamma rays radiation Microwaves Radio waves
High energy 10-15 Wavelength (meters) 10-5 Low 10-11 10-13 10-7 10-9 energy 105 10 10-1 10-3 103 Ultraviolet X-rays Infrared radiation Visible light 400 nm 450 550 500 Violet 600 650 1 = 750 700 Red Copyright © 2009 Pearson Prentice Hall, Inc. a) The first ionization energy of copper metal is 1.24 x 10-18 J. i. ionize the first electron from copper. Determine the type of light source corresponding to the above wavelength. Calculate the maximum wavelength required for light source to be able to i. Gamma rays radiation Microwaves Radio waves
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 2QAP: Most retinal tears and detachments are treated by photocoagulation with a laser. A commonly used...
Related questions
Question
Transcribed Image Text:High
Wavelength
(meters)
energy
Low
10-13
10-15
10-9
10-11
energy
105
10-3
10-5
10-7
103
10
10-1
Ultraviolet
radiation
radiation
Visible light
400 nm
Violet
500
450
650
600
550
A = 750
Red
700
Copyright © 2009 Pearson Prentice Hall, Inc.
a) The first ionization energy of copper metal is 1.24 x 10-18 J.
i.
ionize the first electron from copper.
Determine the type of light source corresponding to the above wavelength.
Calculate the maximum wavelength required for light source to be able to
ii.
Gamma rays
Х-гаys
Infrared
Microwaves
Radio waves
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning