How many ters of a 0.200 M KI solution is needed to completely react with 243 g of Cu(NO,) according to the balanced chemical reaction: KI)-2 Cul(a) + 1(0) KNO,(aq) molCuon peT 0.209 LKI 2.43 0.124 LKI 187.57 CaNon molCuNOT STARTING AMOUNT 2 mol CUNO, 187.57 g Cu(NO,) 1 mol Cu(NO,), 4 mol KI 0.200 LKI 243 g CuNO, * - 0.124 LKI 2 mol KI 0.0010 243 187.57 0.209 0.00542 6.022 * 10" 0.124 0.0620 2 4. 166 o Cu(NO) LKI MKI mal KI mL KI mL Cu(NO,) mol Cu(NO,) L Cu(NO,
How many ters of a 0.200 M KI solution is needed to completely react with 243 g of Cu(NO,) according to the balanced chemical reaction: KI)-2 Cul(a) + 1(0) KNO,(aq) molCuon peT 0.209 LKI 2.43 0.124 LKI 187.57 CaNon molCuNOT STARTING AMOUNT 2 mol CUNO, 187.57 g Cu(NO,) 1 mol Cu(NO,), 4 mol KI 0.200 LKI 243 g CuNO, * - 0.124 LKI 2 mol KI 0.0010 243 187.57 0.209 0.00542 6.022 * 10" 0.124 0.0620 2 4. 166 o Cu(NO) LKI MKI mal KI mL KI mL Cu(NO,) mol Cu(NO,) L Cu(NO,
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 53QAP: The molarity of iodine in solution can be determined by titration with arsenious acid, H3AsO4. The...
Related questions
Question
100%
Please write the conversion factor with the labels. I have a hard time to get a wrong answer on the factor
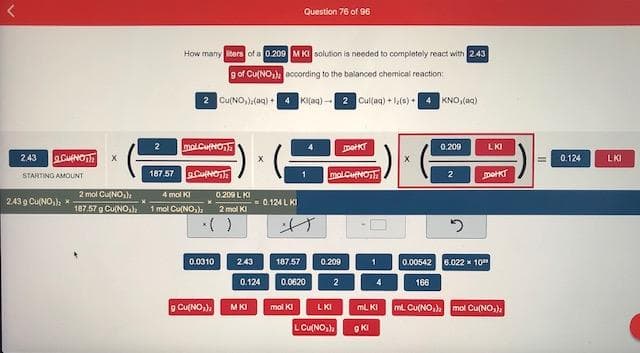
Transcribed Image Text:How many ters of a 0.200 M KI solution is needed to completely react with 243
g of Cu(NO,) according to the balanced chemical reaction:
KI)-2 Cul(a) + 1(0)
KNO,(aq)
molCuon
peT
0.209
LKI
2.43
0.124
LKI
187.57 CaNon
molCuNOT
STARTING AMOUNT
2 mol CUNO,
187.57 g Cu(NO,) 1 mol Cu(NO,),
4 mol KI
0.200 LKI
243 g CuNO, *
- 0.124 LKI
2 mol KI
0.0010
243
187.57
0.209
0.00542
6.022 * 10"
0.124
0.0620
2
4.
166
o Cu(NO)
LKI
MKI
mal KI
mL KI
mL Cu(NO,)
mol Cu(NO,)
L Cu(NO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
