How much 53,5mM potassium hydroxide is needed to bring 47,0mL of a 110,mM solution of dimethylanilinium chloride (pK,= 5,20) to a pH of 4,9. The initial pH of the acid solution is 3,1. less than 10mL between 10 and 15ml O between 15 and 20mL O between 20 and 25mL O between 25 and 30mL O between 30 and 35mL O between 35 and 40mL between 40 and 45mL between 45 and 50mL more than 50mL
How much 53,5mM potassium hydroxide is needed to bring 47,0mL of a 110,mM solution of dimethylanilinium chloride (pK,= 5,20) to a pH of 4,9. The initial pH of the acid solution is 3,1. less than 10mL between 10 and 15ml O between 15 and 20mL O between 20 and 25mL O between 25 and 30mL O between 30 and 35mL O between 35 and 40mL between 40 and 45mL between 45 and 50mL more than 50mL
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 55P
Related questions
Question
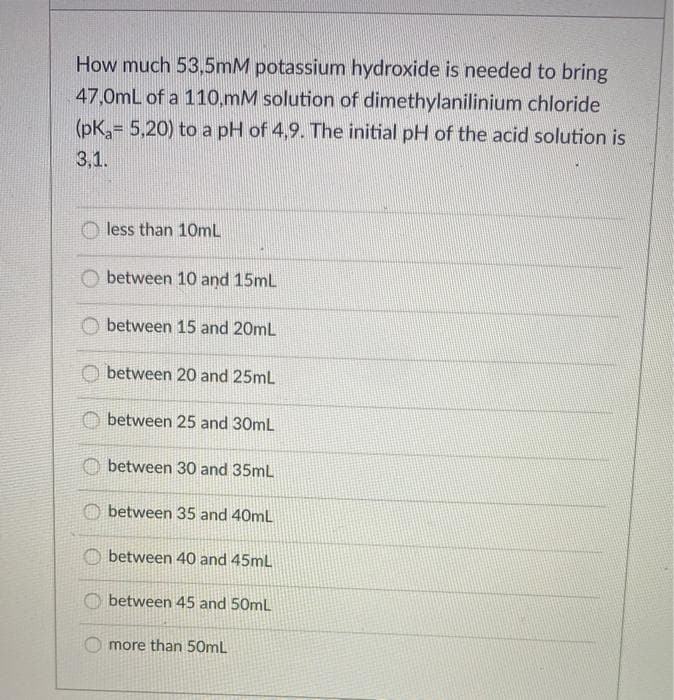
Transcribed Image Text:How much 53,5mM potassium hydroxide is needed to bring
47,0mL of a 110,mM solution of dimethylanilinium chloride
(pK = 5,20) to a pH of 4,9. The initial pH of the acid solution is
3,1.
O less than 10mL
between 10 and 15ml
O between 15 and 20mL
between 20 and 25mL
between 25 and 30mL
O between 30 and 35mL
O between 35 and 40mL
between 40 and 45mL
O between 45 and 50mL
more than 50mL
Expert Solution
Step 1
The molarity of potassium hydroxide is 53.5 mM. The volume of 110 mM dimethylanilinium is 47.0 mL. The initial and the final pH of acid solution is 3.1 and 4.9, respectively. The of dimethylanilinium is 5.20.
Step 2
Convert 53.5 mM to M.
Convert 110 mM to M.
Assume the volume of KOH added is V.
The number of moles of 47.0 mL of 0.11 M dimethylanilinium is calculated as,
The number of moles of 0.0535 M KOH is calculated as,
Step 3
The general chemical reaction between KOH and dimethylanilinium is expressed as,
The above reaction shows that the molar ratio between KOH and dimethylanilinium is 1 : 1. KOH is the limiting reactant.
The ICE table is expressed as,
| Dimethylanilinium | + | KOH | Salt | + | H2O | ||
| I (mol) | 0.00517 | 0 | 0 | ||||
| C (mol) | |||||||
| E (mol) | 0 |
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax