How much heat energy is required to convert 84.0 g (1.82 mol) of liquid ethanol (C2H5OH) at 50.0 °C to gaseous ethanol at 78.4 °C? Melting point of ethanol = Boiling point of ethanol = Molar heat of fusion of ethanol = F114.5 °C 78.4 °C %3D 4.60 kJ/mol Molar heat of vaporization of ethanol = 38.56 kJ/mol Specific heat capacity of liquid ethanol = Specific heat capacity of gaseous ethanol = 1.43 J/g°C 2.45 J/g°C %3D 5.88 x 103 kJ 3.21 x 103 kJ 81.2 kJ 73.4 kJ 1.10 x 104 kJ 76.0 kJ
How much heat energy is required to convert 84.0 g (1.82 mol) of liquid ethanol (C2H5OH) at 50.0 °C to gaseous ethanol at 78.4 °C? Melting point of ethanol = Boiling point of ethanol = Molar heat of fusion of ethanol = F114.5 °C 78.4 °C %3D 4.60 kJ/mol Molar heat of vaporization of ethanol = 38.56 kJ/mol Specific heat capacity of liquid ethanol = Specific heat capacity of gaseous ethanol = 1.43 J/g°C 2.45 J/g°C %3D 5.88 x 103 kJ 3.21 x 103 kJ 81.2 kJ 73.4 kJ 1.10 x 104 kJ 76.0 kJ
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 102E: A 0.250-g chunk of sodium metal is cautiously dropped into a mixture of 50.0 g water and 50.0 g ice,...
Related questions
Question
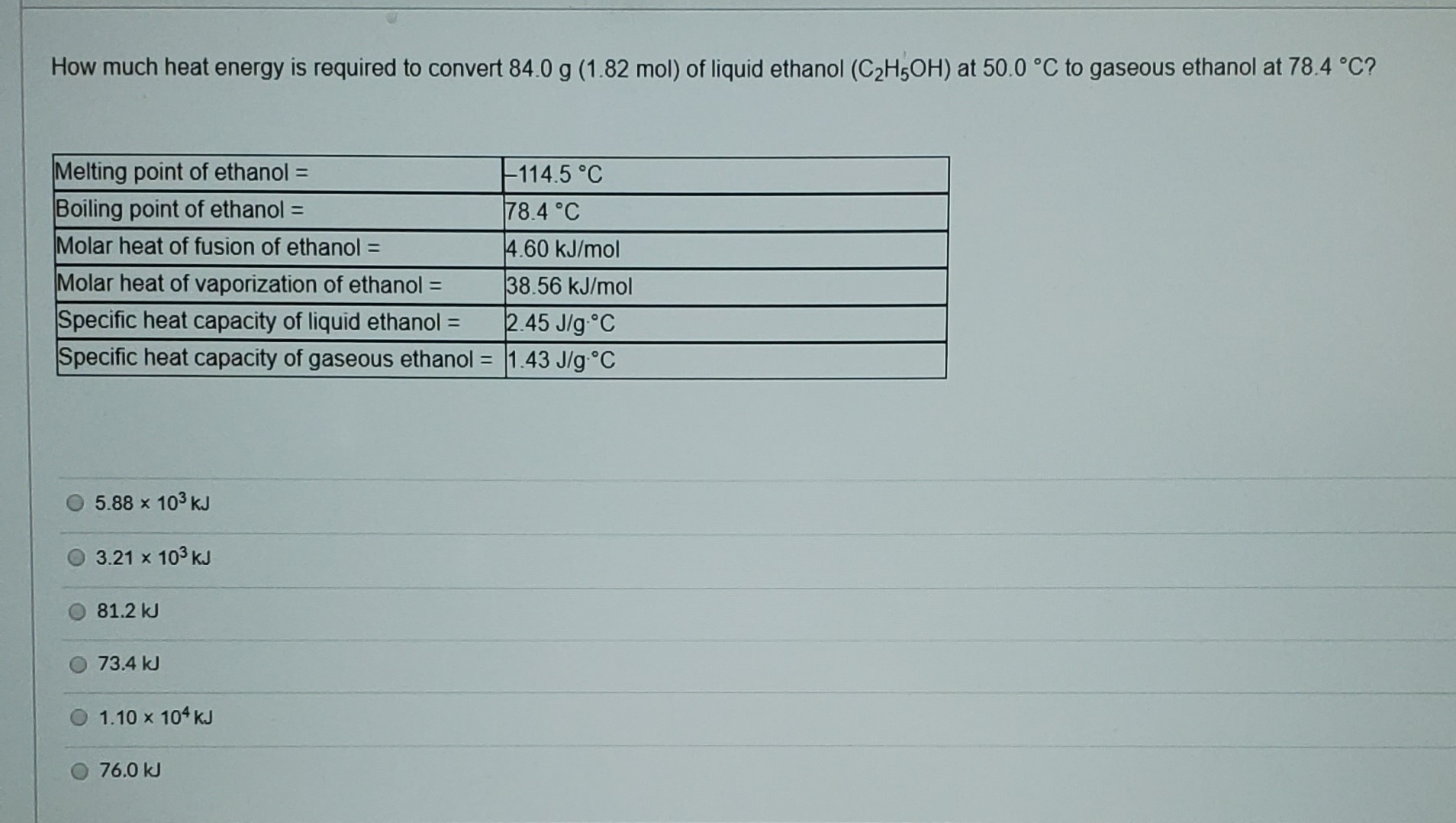
Transcribed Image Text:How much heat energy is required to convert 84.0 g (1.82 mol) of liquid ethanol (C2H5OH) at 50.0 °C to gaseous ethanol at 78.4 °C?
Melting point of ethanol =
Boiling point of ethanol =
Molar heat of fusion of ethanol =
F114.5 °C
78.4 °C
%3D
4.60 kJ/mol
Molar heat of vaporization of ethanol =
38.56 kJ/mol
Specific heat capacity of liquid ethanol =
Specific heat capacity of gaseous ethanol = 1.43 J/g°C
2.45 J/g°C
%3D
5.88 x 103 kJ
3.21 x 103 kJ
81.2 kJ
73.4 kJ
1.10 x 104 kJ
76.0 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning