How much heat would need to be removed to cool 50.3 g of water from 25.6°C to -10.7°C? Csolid = Cliquid = 2.092 J/g.°C 4.184 J/g.°C AHfusion = Tfusion = 0.0 °C %3D 6.01 kJ/mol
How much heat would need to be removed to cool 50.3 g of water from 25.6°C to -10.7°C? Csolid = Cliquid = 2.092 J/g.°C 4.184 J/g.°C AHfusion = Tfusion = 0.0 °C %3D 6.01 kJ/mol
Basic Clinical Laboratory Techniques 6E
6th Edition
ISBN:9781133893943
Author:ESTRIDGE
Publisher:ESTRIDGE
Chapter1: The Clinical Laboratory
Section1.7: The Metric System
Problem 8RQ
Related questions
Question
100%
Please look at image for question! Please help.
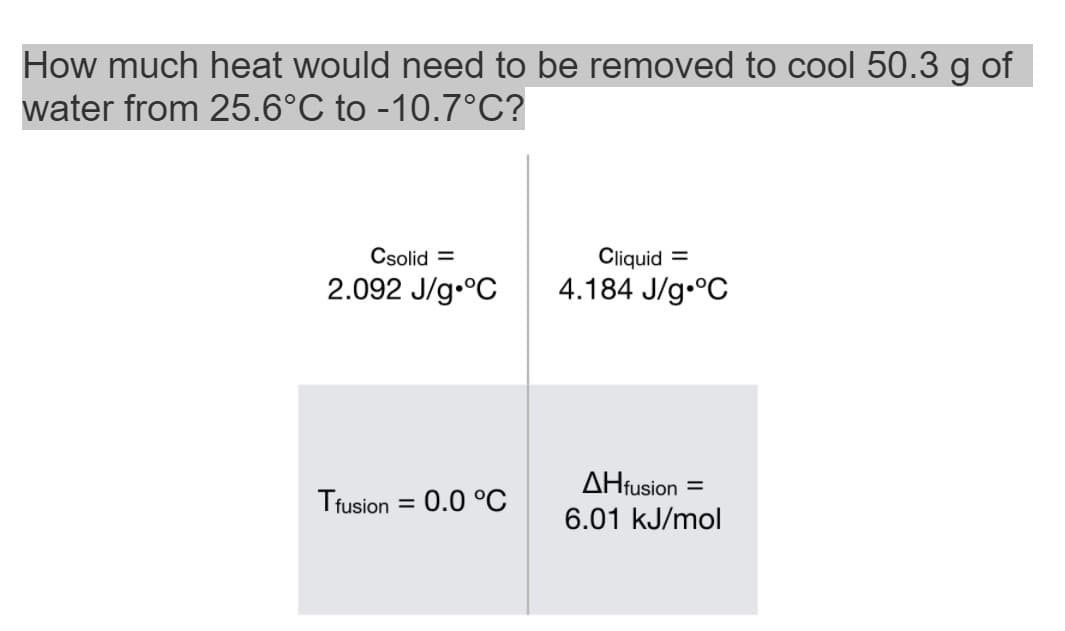
Transcribed Image Text:How much heat would need to be removed to cool 50.3 g of
water from 25.6°C to -10.7°C?
Csolid =
Cliquid
2.092 J/g.°C
4.184 J/g.°C
AHfusion
Tfusion = 0.0 °C
%3D
6.01 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



