How would you prepare 1.00 L of a 0.40-M solution of each of the following? a. H2SO4 from “concentrated" (18 M) sulfuric acid Dilute |mL of concentrated H2SO4 b. HCl from "concentrated" (12 M) reagent to a total volume of 1.00 L with water with 1.00 L water Dilute |mL of concentrated HCl c. NiCl, from the salt NiCl2 · 6H2O Dissolve |g NiCl2 · 6H2O in water, and add water until the total volume of the
How would you prepare 1.00 L of a 0.40-M solution of each of the following? a. H2SO4 from “concentrated" (18 M) sulfuric acid Dilute |mL of concentrated H2SO4 b. HCl from "concentrated" (12 M) reagent to a total volume of 1.00 L with water with 1.00 L water Dilute |mL of concentrated HCl c. NiCl, from the salt NiCl2 · 6H2O Dissolve |g NiCl2 · 6H2O in water, and add water until the total volume of the
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.23QAP
Related questions
Question
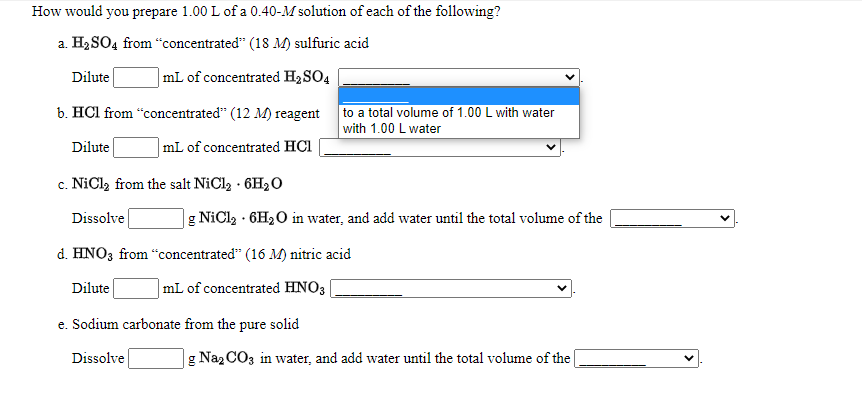
Transcribed Image Text:How would you prepare 1.00 L of a 0.40-M solution of each of the following?
a. H2 SO4 from “concentrated" (18 M) sulfuric acid
Dilute
|mL of concentrated H2SO4
b. HCl from “concentrated" (12 M) reagent
to a total volume of 1.00 L with water
with 1.00 L water
Dilute
mL of concentrated HCl
c. NiCl, from the salt NiCl2 · 6H2O
с.
Dissolve
|g NiCl2 · 6H2O in water, and add water until the total volume of the
d. HNO3 from "concentrated" (16 M) nitric acid
Dilute
mL of concentrated HNO3
e. Sodium carbonate from the pure solid
Dissolve
g Naz CO3 in water, and add water until the total volume of the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning



General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
