Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
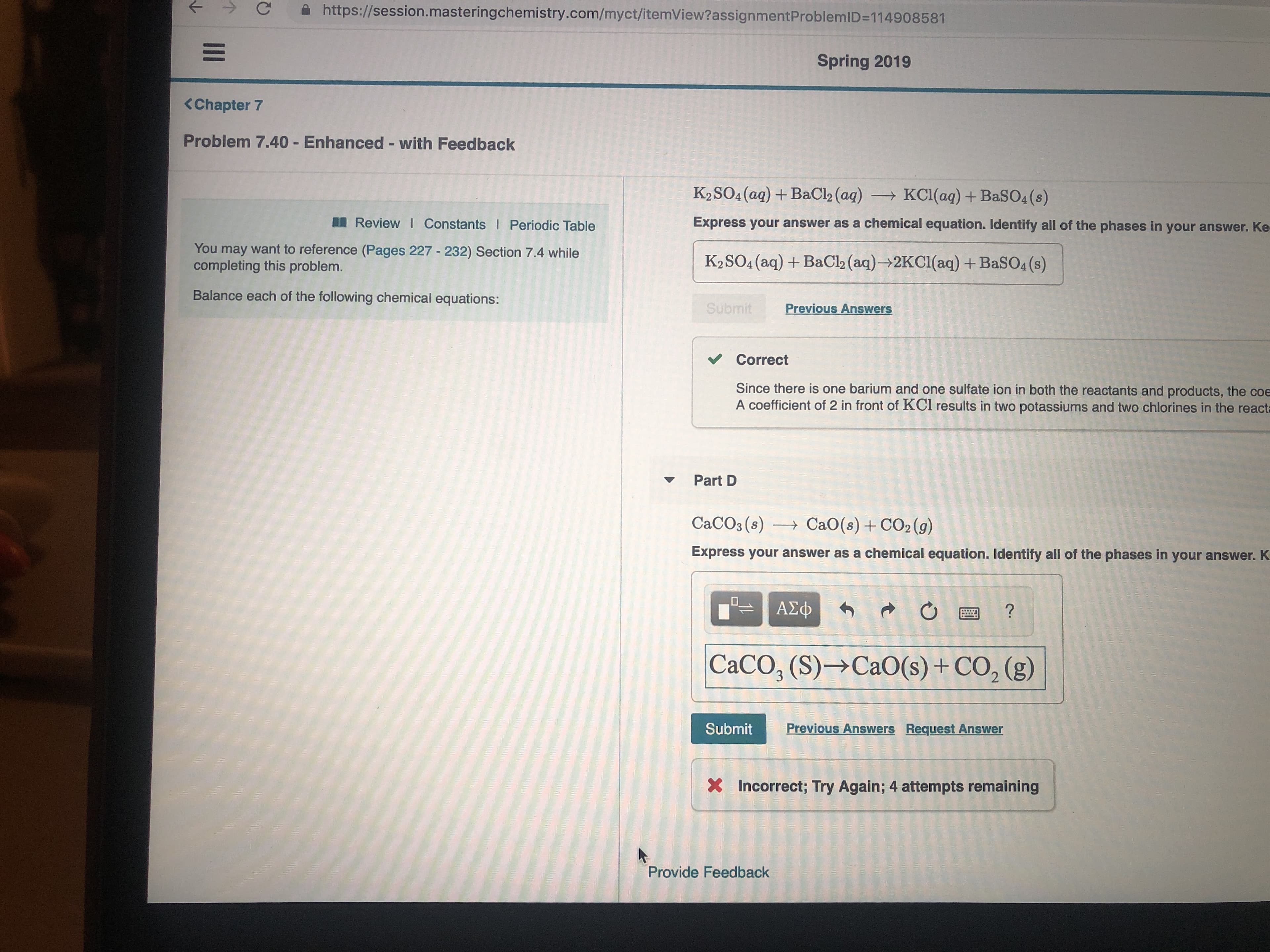
Transcribed Image Text:https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=114908581
Spring 2019
<Chapter 7
Problem 7.40 Enhanced - with Feedback
K2SO4 (aq) BaCl2 (aq)
KCI (aq) + BaSO4 (s)
Express your answer as a chemical equation. Identify all of the phases in your answer. Ke
Review I Constants I Periodic Table
You may want to reference (Pages 227 232) Section 7.4 while
completing this problem.
K2SO4 (aq) BaCl2 (aq)- 2KC1(aq) + BaSO4 (s)
Balance each of the following chemical equations:
Submit
Previous Answers
Correct
Since there is one barium and one sulfate ion in both the reactants and products, the coe
A coefficient of 2 in front of KCl results in two potassiums and two chlorines in the reacta
Part D
CaCO3 (s)
CaO(s)CO2(g)
Express your answer as a chemical equation. Identify all of the phases in your answer. K
ΑΣφ
CaCO, (S)- CaO(s)+CO2 (g)
3
Previous Answers Request Answer
Submit
Incorrect; Try Again; 4 attempts remaining
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY