https://www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8p3jH-US 6JxCWwncg8Tof4a5q5fmgWG31RYRVbH2ANdQdpKMVbYg9DOULcvGibEdaEkBQ508H O THERMOCHEMISTRY Solving a basic calorimetry problem Sc thermometer A 53.5 g sample of aluminum is put into a calorimeter (see sketch at right) that contains 250.0 g of water. The aluminum sample starts off at 91.4 °C and the temperature of the water starts off at 17.0 °C. When the temperature of the water stops changing it's insulated container 20.2 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of aluminum according to this experiment. Be sure your answer is rounded to 2 significant sample digits. a calorimeter J x10 g.°C X Check kplanation Torms of Use Privacy 0 2019 McGraw-Hill Education. All Rights Reserved. 8:09 PM e x O Type here to search 11/20/2019 hp G 1I1
https://www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8p3jH-US 6JxCWwncg8Tof4a5q5fmgWG31RYRVbH2ANdQdpKMVbYg9DOULcvGibEdaEkBQ508H O THERMOCHEMISTRY Solving a basic calorimetry problem Sc thermometer A 53.5 g sample of aluminum is put into a calorimeter (see sketch at right) that contains 250.0 g of water. The aluminum sample starts off at 91.4 °C and the temperature of the water starts off at 17.0 °C. When the temperature of the water stops changing it's insulated container 20.2 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of aluminum according to this experiment. Be sure your answer is rounded to 2 significant sample digits. a calorimeter J x10 g.°C X Check kplanation Torms of Use Privacy 0 2019 McGraw-Hill Education. All Rights Reserved. 8:09 PM e x O Type here to search 11/20/2019 hp G 1I1
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.44E: Biological standard states include specifying a reference temperature of 37.0C rather than the...
Related questions
Question
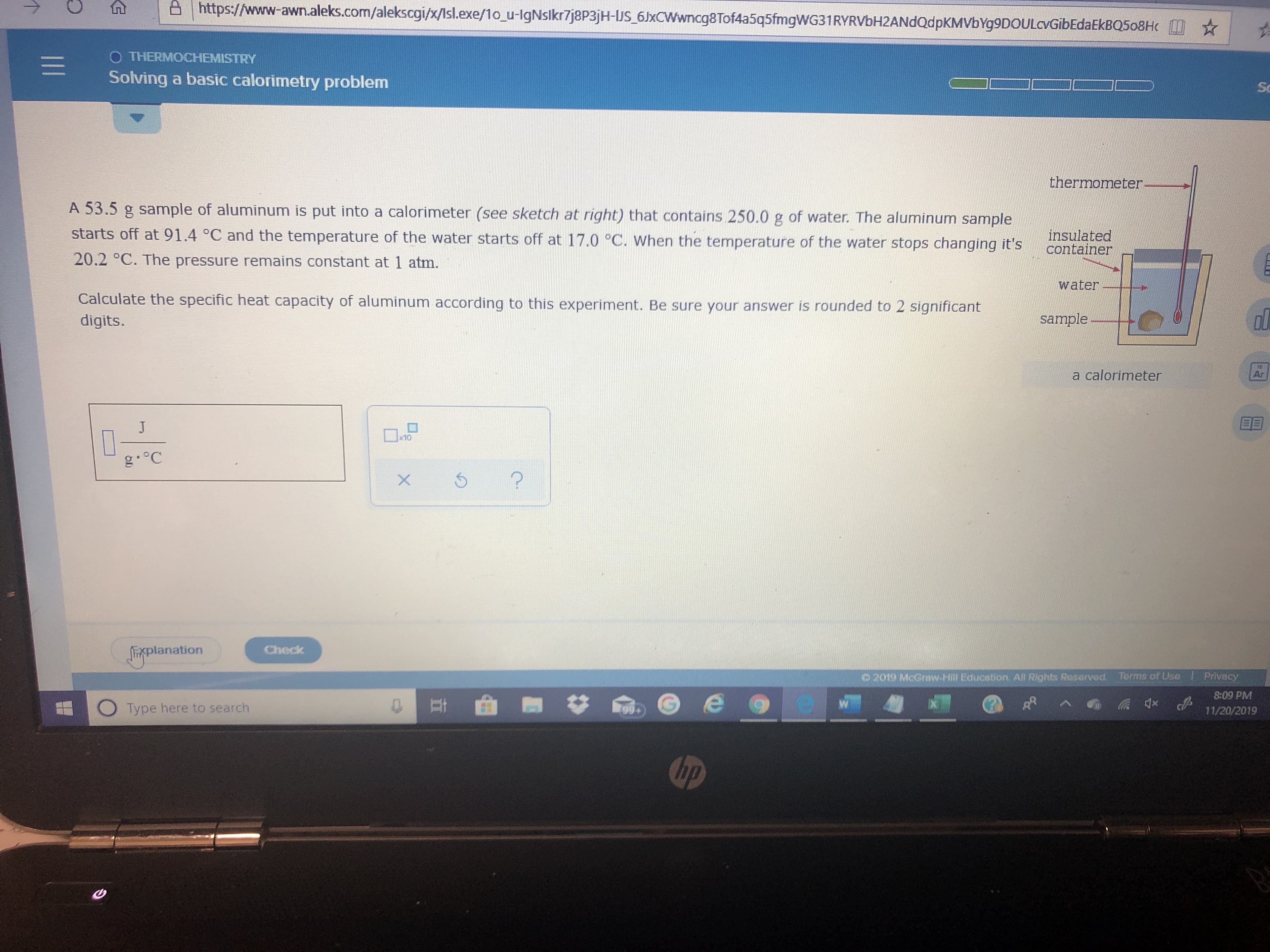
Transcribed Image Text:https://www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8p3jH-US 6JxCWwncg8Tof4a5q5fmgWG31RYRVbH2ANdQdpKMVbYg9DOULcvGibEdaEkBQ508H
O THERMOCHEMISTRY
Solving a basic calorimetry problem
Sc
thermometer
A 53.5 g sample of aluminum is put into a calorimeter (see sketch at right) that contains 250.0 g of water. The aluminum sample
starts off at 91.4 °C and the temperature of the water starts off at 17.0 °C. When the temperature of the water stops changing it's
insulated
container
20.2 °C. The pressure remains constant at 1 atm.
water
Calculate the specific heat capacity of aluminum according to this experiment. Be sure your answer is rounded to 2 significant
sample
digits.
a calorimeter
J
x10
g.°C
X
Check
kplanation
Torms of Use Privacy
0 2019 McGraw-Hill Education. All Rights Reserved.
8:09 PM
e
x
O Type here to search
11/20/2019
hp
G
1I1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div