I Medical X Course Home + X .ecollege.com/course.html?courseld =15556158&HepID=6b9f9b2f... 2 012 Chapter 9A Problem 1 > 1 of 14 Part B A solution of rubbing alcohol is 71.9 % (v/v) isopropanol in water. How many milliliters of isopropanol are in a 92.5 mL sample of the rubbing alcohol solution? Express your answer to three significant figures. View Available Hint(s) ? ΑΣφ mL Milliliters of isopropanol Submit Part C How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L. t
I Medical X Course Home + X .ecollege.com/course.html?courseld =15556158&HepID=6b9f9b2f... 2 012 Chapter 9A Problem 1 > 1 of 14 Part B A solution of rubbing alcohol is 71.9 % (v/v) isopropanol in water. How many milliliters of isopropanol are in a 92.5 mL sample of the rubbing alcohol solution? Express your answer to three significant figures. View Available Hint(s) ? ΑΣφ mL Milliliters of isopropanol Submit Part C How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L. t
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.44E
Related questions
Question
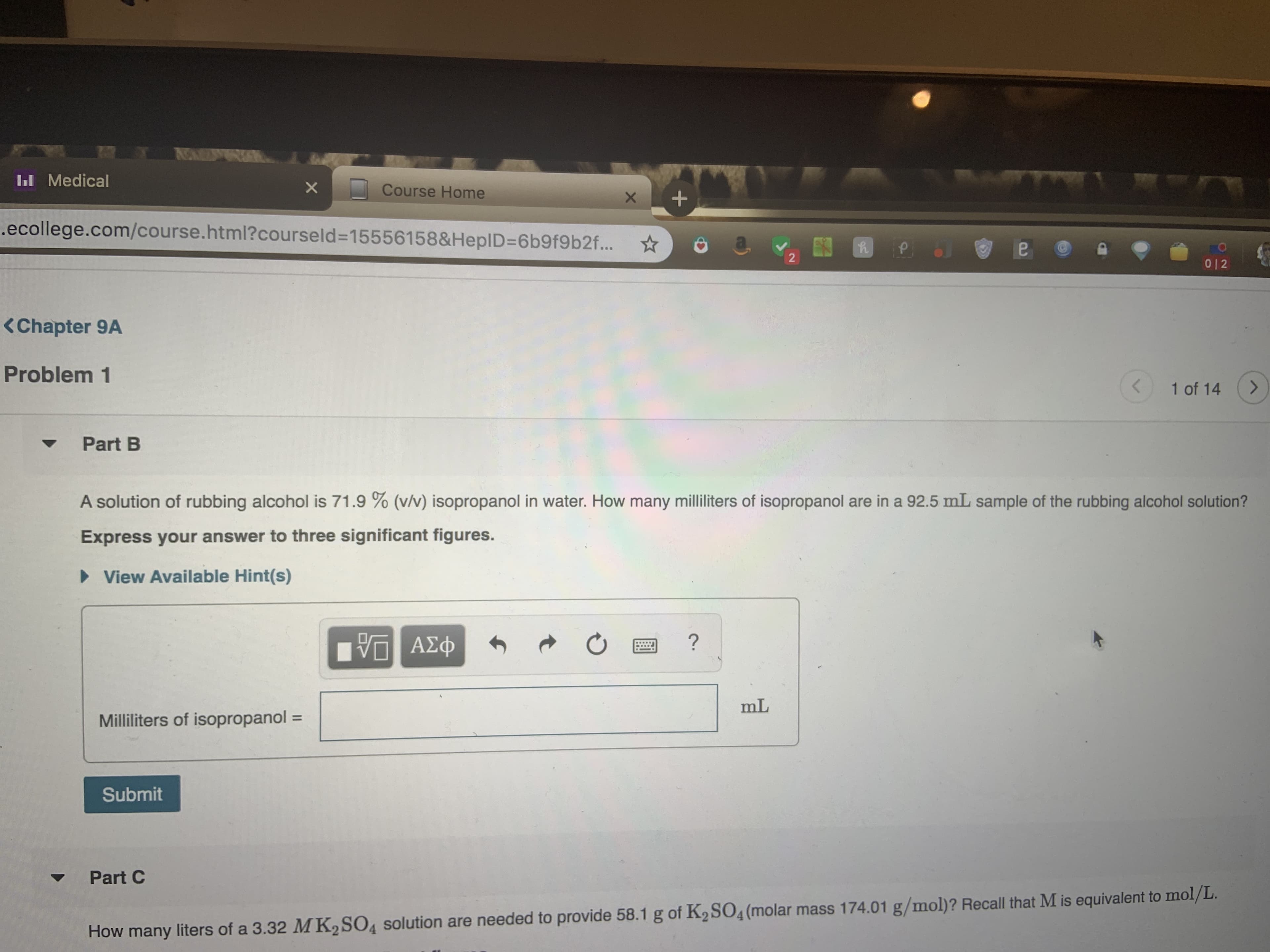
Transcribed Image Text:I Medical
X
Course Home
+
X
.ecollege.com/course.html?courseld =15556158&HepID=6b9f9b2f...
2
012
Chapter 9A
Problem 1
>
1 of 14
Part B
A solution of rubbing alcohol is 71.9 % (v/v) isopropanol in water. How many milliliters of isopropanol are in a 92.5 mL sample of the rubbing alcohol solution?
Express your answer to three significant figures.
View Available Hint(s)
?
ΑΣφ
mL
Milliliters of isopropanol
Submit
Part C
How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L.
t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Recommended textbooks for you



