I Review I Constants I P III LI IS LILI alivin, ti e connceIILI aliun UI Dase iS KNOWN aliu Can Ue useu LU Caiculale e uiKN Learning Goal: concentration: To calculate the concentration of a solution using moles of base moles of acid concentration of base concentr acid-base titration data. In an acid-base titration an acid (or base) of known concentration is aaded to a base (or acid) of unknown concentration until the number of moles of H and OH are equal, a condition called the equivalence point. Since you know the number of moles of HT (or OH) that you added, you can Part A How many moles of Ba(OH)2 are present in 205 mL of 0.600 M Ba(OH),? Express your answer with the appropriate units. determine the number of moles of OH (or H) in View Available Hint(s) the unknown solution. For example, a solution containing 1 mol of H2 SO4 contains 2 mol of ionizable hydrogen atoms, and would therefore require 2 mol of NaOH for neutralization. A ? M 0.123 Previous Answers Request Answer Submit Incorrect; Try Again; 4 attempts remaining X Enter your answer using units of amount of substance. P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy, I Permissions | Contact
I Review I Constants I P III LI IS LILI alivin, ti e connceIILI aliun UI Dase iS KNOWN aliu Can Ue useu LU Caiculale e uiKN Learning Goal: concentration: To calculate the concentration of a solution using moles of base moles of acid concentration of base concentr acid-base titration data. In an acid-base titration an acid (or base) of known concentration is aaded to a base (or acid) of unknown concentration until the number of moles of H and OH are equal, a condition called the equivalence point. Since you know the number of moles of HT (or OH) that you added, you can Part A How many moles of Ba(OH)2 are present in 205 mL of 0.600 M Ba(OH),? Express your answer with the appropriate units. determine the number of moles of OH (or H) in View Available Hint(s) the unknown solution. For example, a solution containing 1 mol of H2 SO4 contains 2 mol of ionizable hydrogen atoms, and would therefore require 2 mol of NaOH for neutralization. A ? M 0.123 Previous Answers Request Answer Submit Incorrect; Try Again; 4 attempts remaining X Enter your answer using units of amount of substance. P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy, I Permissions | Contact
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 102AP
Related questions
Question
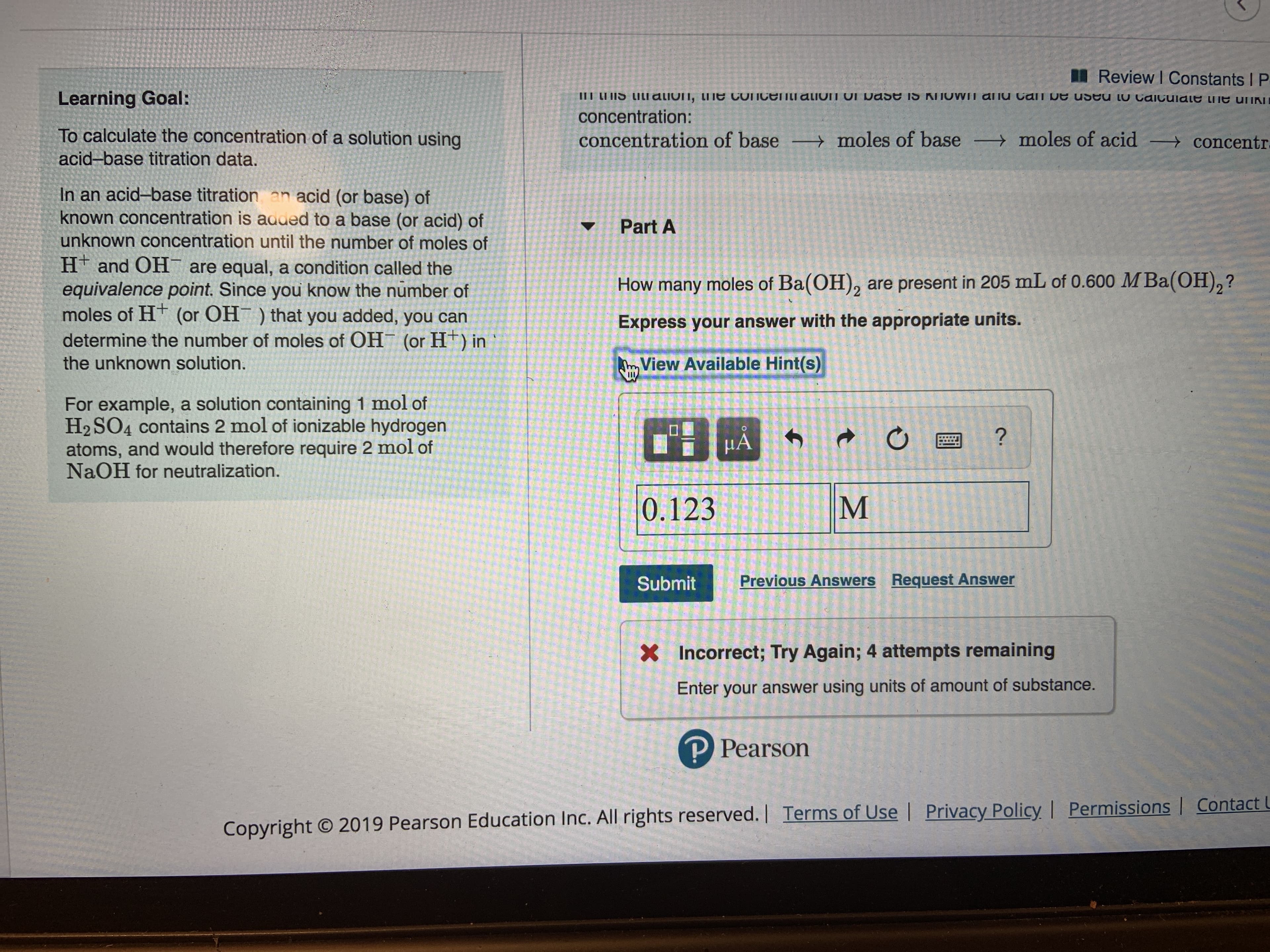
Transcribed Image Text:I Review I Constants I P
III LI IS LILI alivin, ti e connceIILI aliun UI Dase iS KNOWN aliu Can Ue useu LU Caiculale e uiKN
Learning Goal:
concentration:
To calculate the concentration of a solution using
moles of base moles of acid
concentration of base
concentr
acid-base titration data.
In an acid-base titration an acid (or base) of
known concentration is aaded to a base (or acid) of
unknown concentration until the number of moles of
H and OH are equal, a condition called the
equivalence point. Since you know the number of
moles of HT (or OH) that you added, you can
Part A
How many moles of Ba(OH)2 are present in 205 mL of 0.600 M Ba(OH),?
Express your answer with the appropriate units.
determine the number of moles of OH
(or H) in
View Available Hint(s)
the unknown solution.
For example, a solution containing 1 mol of
H2 SO4 contains 2 mol of ionizable hydrogen
atoms, and would therefore require 2 mol of
NaOH for neutralization.
A
?
M
0.123
Previous Answers Request Answer
Submit
Incorrect; Try Again; 4 attempts remaining
X
Enter your answer using units of amount of substance.
P Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy, I Permissions | Contact
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning