I. Pressure, Volume Relationship The relationship between pressure and volume of a gas is given by Boyle's Law (PV = k). A sample gas trapped in a large calibrated syringe can have pressure applied to it. Using an apparatus of this sort, the following data was obtained: Pressure (atm) Volume of trapped gas (mL) 0.50 500 0.60 400 0.75 300 1.2 200 2.3 100 4.5 50 (a) Using Excel, prepare a graph of V vs. P using the data above. Include all of the pertinent data and components that must accompany this graph as described in the Background Video. (b) Using Excel, prepare a graph of 1/V vs. P using the same data. Include all of the pertinent data and components that must accompany this graph as described in the Background Video. (c) State which graph is more linear and explain how you know this. (Hint: Any time you are asked to explain your reasoning in a lab course, use numerical values to support your explanation.) (d) If you were asked to report the slope of the line generated in either graph (a) or (b), how many significant figures would make sense to report? Explain your reasoning.
I. Pressure, Volume Relationship The relationship between pressure and volume of a gas is given by Boyle's Law (PV = k). A sample gas trapped in a large calibrated syringe can have pressure applied to it. Using an apparatus of this sort, the following data was obtained: Pressure (atm) Volume of trapped gas (mL) 0.50 500 0.60 400 0.75 300 1.2 200 2.3 100 4.5 50 (a) Using Excel, prepare a graph of V vs. P using the data above. Include all of the pertinent data and components that must accompany this graph as described in the Background Video. (b) Using Excel, prepare a graph of 1/V vs. P using the same data. Include all of the pertinent data and components that must accompany this graph as described in the Background Video. (c) State which graph is more linear and explain how you know this. (Hint: Any time you are asked to explain your reasoning in a lab course, use numerical values to support your explanation.) (d) If you were asked to report the slope of the line generated in either graph (a) or (b), how many significant figures would make sense to report? Explain your reasoning.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
Help me
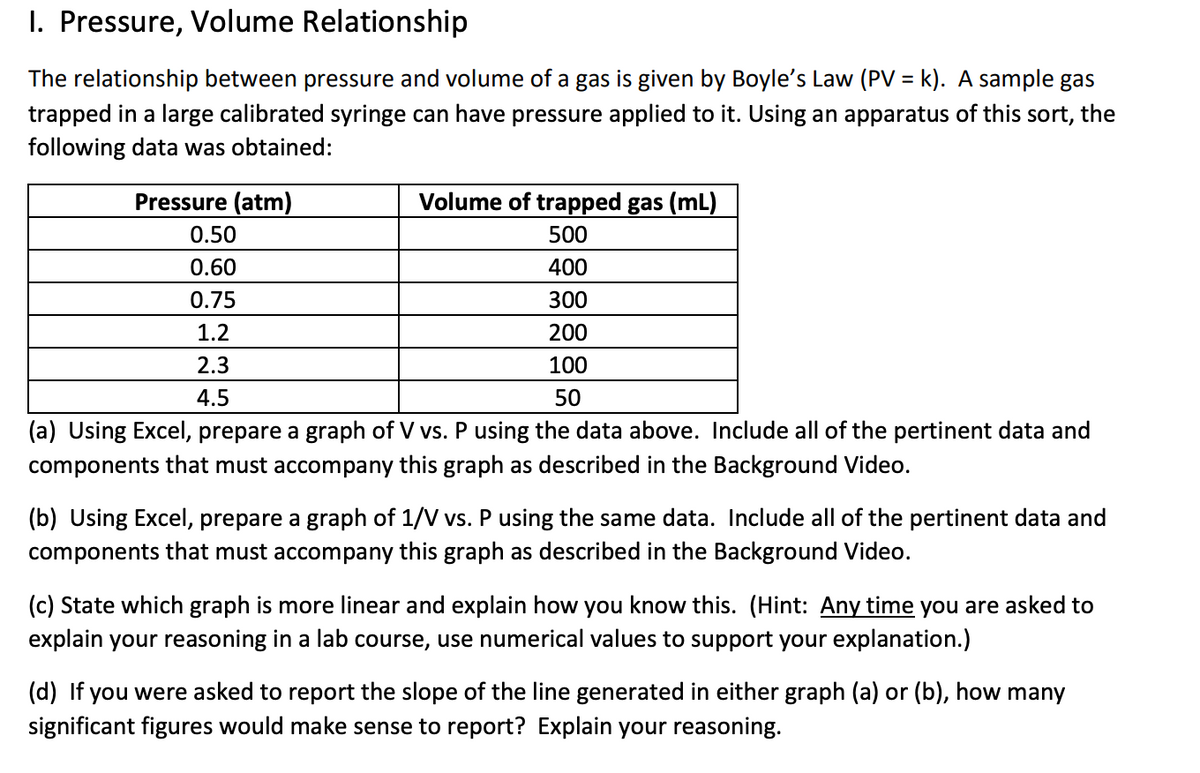
Transcribed Image Text:I. Pressure, Volume Relationship
The relationship between pressure and volume of a gas is given by Boyle's Law (PV = k). A sample gas
trapped in a large calibrated syringe can have pressure applied to it. Using an apparatus of this sort, the
following data was obtained:
%3D
Pressure (atm)
Volume of trapped gas (mL)
0.50
500
0.60
400
0.75
300
1.2
200
2.3
100
4.5
50
(a) Using Excel, prepare a graph of V vs. P using the data above. Include all of the pertinent data and
components that must accompany this graph as described in the Background Video.
(b) Using Excel, prepare a graph of 1/V vs. P using the same data. Include all of the pertinent data and
components that must accompany this graph as described in the Background Video.
(c) State which graph is more linear and explain how you know this. (Hint: Any time you are asked to
explain your reasoning in a lab course, use numerical values to support your explanation.)
(d) If you were asked to report the slope of the line generated in either graph (a) or (b), how many
significant figures would make sense to report? Explain your reasoning.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax