If 10.27 kJ of heat is given off when 51.93 g of unknown acid condenses from vapor to liquid, what is AHcond for this substance? The corresponding molecular weight is 45.16 g/mol.
If 10.27 kJ of heat is given off when 51.93 g of unknown acid condenses from vapor to liquid, what is AHcond for this substance? The corresponding molecular weight is 45.16 g/mol.
Chapter10: Energy
Section: Chapter Questions
Problem 50A
Related questions
Question
use the second image as reference for standards
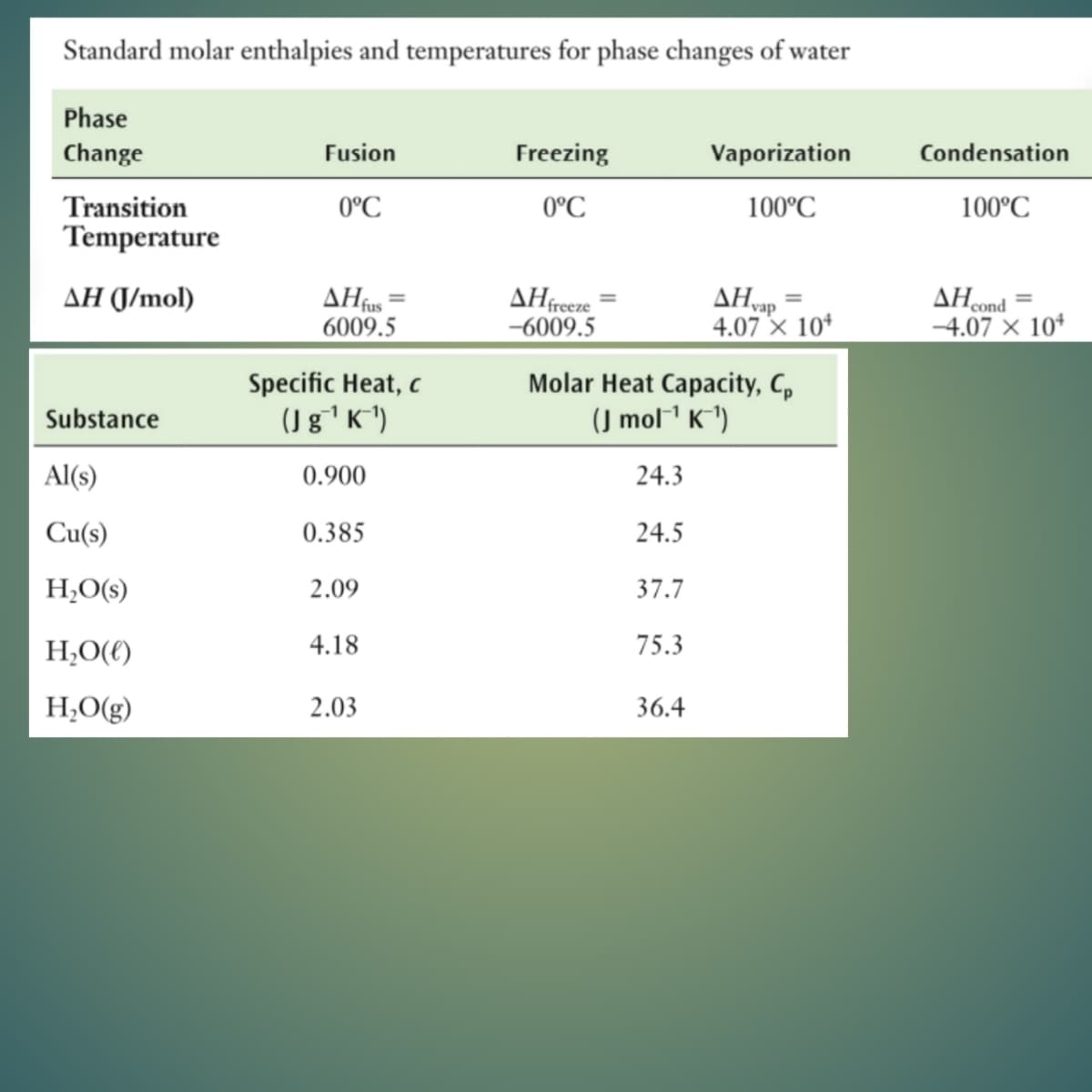
Transcribed Image Text:Standard molar enthalpies and temperatures for phase changes of water
Phase
Change
Fusion
Freezing
Vaporization
Condensation
Transition
0°C
0°C
100°C
100°C
Temperature
ΔΗ
6009.5
AHvap
4.07 × 10*
AHcond
-4.07 × 10*
AH (J/mol)
AHfreeze
-6009.5
Specific Heat, c
(Jg'K*)
Molar Heat Capacity, C,
(J mol1 K^1)
Substance
Al(s)
0.900
24.3
Cu(s)
0.385
24.5
H;O(s)
2.09
37.7
H,O(()
4.18
75.3
H;O(g)
2.03
36.4

Transcribed Image Text:If 10.27 kJ of heat is given off when 51.93 g of unknown acid condenses from vapor to liquid, what is AHcond
for this substance? The corresponding molecular weight is 45.16 g/mol.
Round your answer to 2 decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning