If a sample of BaBrz is found to contain 2.34 · 103 mol of Ba2+ ions how many moles of Br" ions are present? Express your answer in scientific notation. x 10
If a sample of BaBrz is found to contain 2.34 · 103 mol of Ba2+ ions how many moles of Br" ions are present? Express your answer in scientific notation. x 10
Chapter4: Molecules, Compounds, And Chemical Reactions
Section: Chapter Questions
Problem 4SC
Related questions
Question
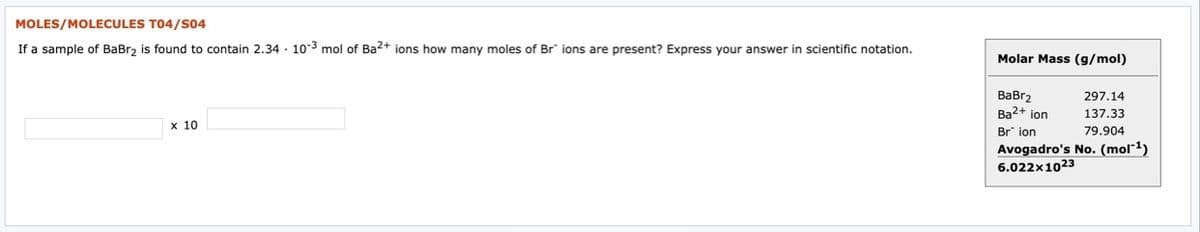
Transcribed Image Text:MOLES/MOLECULES T04/S04
If a sample of BaBr, is found to contain 2.34 · 10-3 mol of Ba2+ ions how many moles of Br" ions are present? Express your answer in scientific notation.
Molar Mass (g/mol)
ВаBrz
Ba2+ ion
297.14
137.33
х 10
Br ion
79.904
Avogadro's No. (mol"1)
6.022x1023
Expert Solution
Step 1
1 mole of BaBr2 after 100% dissociation produces 1 mol Ba2+ ions and 2 mol Br- ions
BaBr2 → Ba2+ + 2 Br-
Number of mole of Br- ions = 2 Number of mole of Ba2+ ions.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning