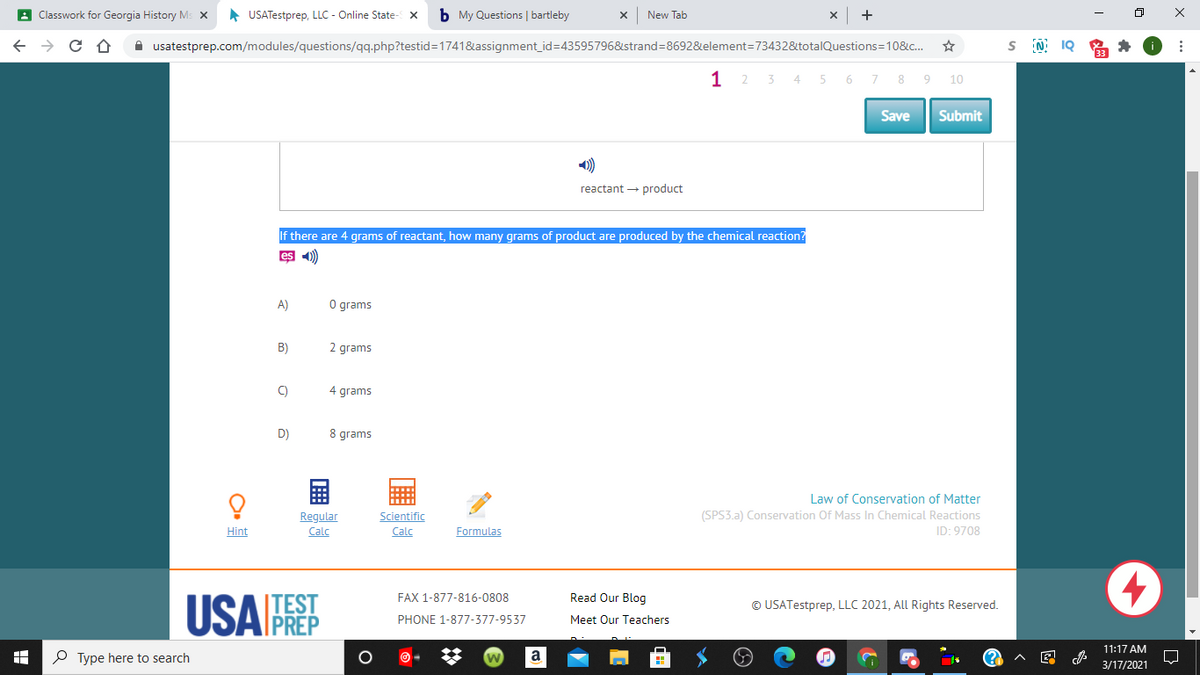
If there are 4 grams of reactant, how many grams of product are produced by the chemical reaction? A) O grams B) 2 grams C) 4 grams D) 8 grams
If there are 4 grams of reactant, how many grams of product are produced by the chemical reaction? A) O grams B) 2 grams C) 4 grams D) 8 grams
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 5CR
Related questions
Question
Transcribed Image Text:A Classwork for Georgia History Ms X
A USATestprep, LLC - Online State- x
b My Questions | bartleby
New Tab
+
A usatestprep.com/modules/questions/qq.php?testid=1741&assignment_id=43595796&strand=8692&element=73432&totalQuestions=10&c.
S N: 1Q
1
2 3
4 5
7
8
9
10
Save
Submit
reactant - product
If there are 4 grams of reactant, how many grams of product are produced by the chemical reaction?
es 4)
A)
O grams
B)
2 grams
C)
4 grams
D)
8 grams
Law of Conservation of Matter
(SPS3.a) Conservation Of Mass In Chemical Reactions
Regular
Calc
Scientific
Hint
Calc
Formulas
ID: 9708
USA PREP
FAX 1-877-816-0808
Read Our Blog
© USATestprep, LLC 2021, All Rights Reserved.
PHONE 1-877-377-9537
Meet Our Teachers
11:17 AM
P Type here to search
a
3/17/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax