Imagine a titration of 38.77 mg of a solid dissolved in water. If the titration is stopped when pAg=8.04 and 1.293 mL of 0.1000 M AGNO3 titrant has been added. What is the percent KI in the original solid sample? The molar mass of Kl is 166.0028 g/mol. percent KI=
Q: 0.400 M
A: A) mmoles of Acid = 60.0mL • 0.355molL-1 = 21.3mmol B) mmles of Base = 75.0mL • 0.400molL-1 =…
Q: Two solutions of an unknown slightly soluble salt, A(OH)2, were allowed to equilibrate—one at 25 °C…
A: Given: Volume of A(OH)2 =15 mL Concentration of HCl = 0.20 M At 25oC Volume of HCl =6.37 mL At 80oC…
Q: Grape juice contains diprotic acid, tartaric acid, which has the molecular formula C4H6O6. If 20.00…
A:
Q: Molarity of the standard solution of sodium hydroxide = 0.1010 mol/L Volumes of NaOH(aq): Second…
A: This titration involves the neutralization of an acid by NaOH.
Q: Calculate the molar concentration of a dilute Ba(OH)2 solution if addition of 50.00mL of the base…
A:
Q: Consider the titration of 50.0 mL of 0.501 M weak base B (Kb = 7.5 x 10⁻⁶) with 0.340 M HNO₃. What…
A:
Q: Consider the reaction of a 20.0 mL of 0.220 M C₅H₅NHCl (Ka = 5.9 x 10⁻⁶) with 12.0 mL of 0.211 M…
A: Explanation: Molarity is defined as moles of solute, which in your case is sodium hydroxide,…
Q: Consider the titration of a 50.0-mL sample of 0.0500 M HCI with a 0.0250 M NaOH solution. What is…
A:
Q: Consider the titration of a 60.0 mL of 0.291 M weak acid HA (Ka = 4.2 x 10⁻⁶) with 0.400 M KOH. What…
A: The question is based on the concept of titrations. we have been given a weak Acid which is titrated…
Q: A 0.3654 g portion of pure formic acid (CH2O2, FW=46.03 g/mol, Ka=1.77 x 10-4) is dissolved in 50.00…
A:
Q: If you have a 74.0 mL solution of 0.160 M benzoic acid, C6H5 COOH, and it is being titrated with…
A: Given: Concentration of benzoic acid (Assuming HA) = 0.160 M Volume of HA solution = 74.0 mL = 0.074…
Q: 25.00 mL of 0.109 M benzoic acid (Ka = 6.5 x 10-5 ) is titrated with 0.134 M sodium hydroxide. What…
A:
Q: Based on the tritration curve shown below, what's the pKa of the acid being titrated? Titration of a…
A: Titration curve for a titration of a weak acid with a strong base is given.
Q: Consider the titration of 20.00 mL of 0.1000 M butanoic acid (C3H7COOH) (Ka = 1.54 x 10), with…
A:
Q: Calculate the molar concentration of a dilute Ba(OH)2 solution if addition of 50.00 mL of the base…
A: Given information: Mass of benzoic acid = 0.3912 g Volume of the solution = 50.00 mL Volume of HCl =…
Q: Calculate the pH during the titration of 40.00 mL of 0.0250 M KOH with 0.0500 M HCl after addition…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: In a titration of 35.00mL of 0.737 M H2SO4 , ___mL of a 0.827 M KOH solution is required for…
A:
Q: Do the argentometric titration according to given data and fill in the blanks. Ag+ (aq) + Cl- (aq) →…
A: The titration of one solution with another helps to calculate the concentration of the unknown…
Q: Consider the titration of 50.0 mL of 0.425 M weak base B (Kb = 7.5 x 10⁻⁶) with 0.340 M HNO₃. What…
A:
Q: Show that the pH at the halfway point is equivalent to titration of a weak acid with a strong base…
A: The reaction of a weak acid with strong base will be like HA + BOH -------> BA + H2O where HA =…
Q: Consider the titration of 150.0 mL of a 0.0040 M solution of the weak base caffeine (C8H₁0N4O2, K₂ =…
A:
Q: What is the function of the vacuum aspirator? Predict the color shift of the indicator as a…
A: Aspirator is a tool which uses suction to remove or collect something specially in medicine for…
Q: In titrating 10 ml of 0.1N HA (pKa=4),the pH after the addition NaOH is O 2.32 3.25 O 3.61 4.50 4.36
A: Buffer is the solution of mixture of weak acid/weak base with its salt of strong base/strong acid
Q: Consider the titration of 100.0 mL of 0.250 M aniline, C6H5NH2, (Kp = 3.8 x 10-10) with 0.500 M HCI…
A: Here concentration of aniline is given 0.250M... And we know that pH + pOH = 14...
Q: The molarity of a 10.00 mL aliquot of an unknown monoprotic acid titrated with 13.37 mL of 1.25 M…
A: Formula :- Macid x Vacid = Mbase x Vbase To calculate :- Macid
Q: A plot of pH vs volume of 0.05 M NaOH solution was obtained for titrating 0.4055 g of a dried solid…
A: The plot of pH vs volume of 0.05 M NaOH solution given is as,
Q: The concentration of CO2 in air may be determined by an indirect acid-base titration. A sample of…
A: Molarity - ratio of number of moles of solute to the volume of solution in litres. Formula is,
Q: Consider the titration of 24.6 ml of 0.200 M HNO2 (Ka = 7.1×10-4) with 0.519 M KOH. What is the…
A: The equivalence point is the point in a titration where the amount of titrant added completely…
Q: 4. In an experiment to determine the concentration of an HCCI sample of unknown concentration, 0.1 M…
A: The concentration of unknown solutions is calculated by using the process of titration. The…
Q: The pH value for the titration of 50ml of 0.1N CH3COOH, Ka =10-5 with 25 ml of 0.2N NAOH is: *
A: Given : Reaction between acetic acid and NaOH CH3COOH + NaOH <------------> CH3COONa +…
Q: Calculate the molarity of NaOH (40 g/mol) solution if 12.25 mL was used to titrate 0.2615 gram of…
A:
Q: The half-equivalence point of a titration occurs half way to the equivalence point, where half of…
A:
Q: In a titration 36.7 mL of HBr solution is put In a flask with a few drops of phenolphthalein and…
A: Given: The volume of HBr is 36.7 mL. The volume of Sr(OH)2 is 51.4 mL. The molarity of Sr(OH)2 is…
Q: A student set up a titration apparatus that involved using 25.9 mL of 1.57 M acetic acid. The acetic…
A: Given : volume of acetic acid = 25.9 ml Molarity of acetic acid = 1.57 M Molarity of NaOH =0.154 M…
Q: What is the volume of added acid at the equivalence point for KOH? Two 19.0 mL samples, one 0.200 M…
A: Part A :- Volume of acid at the equivalence point will be equal to for KOH = 38 ml
Q: 2. Draw a graph of pH vs Vh for the titration of a strong acid with a strong base and compare it to…
A:
Q: 1. What is the effect on the computed molarity of KMNO4 if you stop titrating when the titrate…
A: Titration of oxalic with KMnO4 is an example of redox reaction with permanent pink colour as the end…
Q: Consider the titration of 130. mL of 0.355 M HCI with 0.275 M NaOH. What is pH after 115 mL of NaOH…
A: Given Volume of HCl (V1) = 130. mL Molarity of HCl (M1) = 0.355 M Volume of NaOH (V2) = 115 mL…
Q: Generate a titration curve for 25.0 mL of 0.7233 M H;POs, with 1.256 M NAOH as the titrant, given…
A: The titration curve for the titration of 25.0 mL of 0.7233 M H3PO4 versus 1.256 M NaOH solution is…
Q: Molarity of the standard solution of sodium hydroxide = 0.1010 mol/L Volumes of NaOH(aq): Rough…
A: In the given question, molarity of the NaOH solution is given. Molarity = 0.1010 mol/L The initial…
Q: Consider the titration of 100.0 mL of 0.100 M H2NNH2 (Kb = 3.0 × 10−6 ) by 0.200 M HNO3. Calculate…
A: Hii there, As there are multiple sub parts posted. we are answering first-three sub parts . If you…
Q: Derive the titration curve of the following solution: a. 20.00 mL, 0.25 M CaCO, with 0.20 M AgNOs…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate the pH of a solution containing 19 mL of 0.93 M of a weak acid AH, for instace CH3COOH,…
A:
Q: Choose from the list below the possible indicators for the titration of 25 mL of a 0.1000 mol L^-1…
A: Volumetric titration involves determination of concentration of an analyte by measuring the volumes…
Q: Consider the titrimetric determination of acetic acid (60.05 g/mol) in vinegar. Titration of a 5.00…
A: Given: Acetic acid titration with NaOH. And the solution is at the point which is before the…
Q: Consider the titration of 300.0 mL of 0.500 M NH3 (Kh = 1.8 x 105) with 0.500 M HNO3. What is the pH…
A: Mnmn
Q: Molarity of the standard solution of sodium hydroxide = 0.1010 mol/L Volumes of NaOH(aq): Rough…
A: In the given question, molarity of NaOH is given which is 0.1010 mol/L. In the table of column,…
Q: Consider the titration of a 60.0 mL of 0.355 M weak acid HA (Ka = 4.2 x 10⁻⁶) with 0.400 M KOH.…
A:
Q: Consider the titrimetric determination of acetic acid (60.05 g/mol) in vinegar. Titration of a 5.00…
A:
Q: Determine the pH at the point in the titration of 40.0 mL of 0.200 M HC4H,O2 with 0.100 M Sr(OH)2…
A: Acid-base titration is an analytical technique utilized to determine the unknown concentration of an…
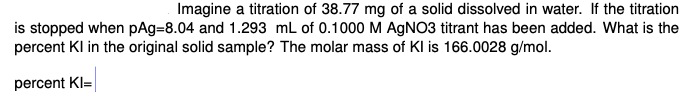
Step by step
Solved in 6 steps

- What mass of Ba(OH)2 is present in a sample if it is titrated to its equivalence point with 44.20 mL of 0.1000 N H2SO4? Note: Present complete solutions for the following problem. Express your final answers up to two (2) decimal places.What is the solubility of silver iodide in grams per milliliter at a temperature at which the Ksp of AgI is 1.52×10-16?Consider the titrimetric determination of acetic acid (60.05 g/mol) in vinegar. Titration of a 5.00 mL vinegar sample requires 12.15 mL of 0.250 M NaOH to reach the phenolphthalein endpoint. How many grams of acetic acid are present in the sample?
- A 25 mL solution of 0.255 M AgNO3 titrant was added to a 50 mL sample that contains chromate ion (CrO4 2-). It required 12.39 mL of a 0.1998 M thiocyanate (SCN- ) solution to titrate to a red point. What is the concentration of the chromate ion in ppm and molarity?Neutralization Titration In the standardization of HCI solution using 10.00 ml of 0.02 Na,co, according to the equation M of Na,CO, 2 HCI 2NaCl + H₂O+CO, 15.0 ml of HCI is required to reach the end point using Bromocresol green indicator the molarity of HCI (mol/L) would be al 0.032 b) 0.027 20.013 d) 0.008 e) 0.06410- A solution with a volume of 100 ml is prepared by weighing 2.50 g from the mixture of Kl and KBr. When 10 ml of this solution is titrated with 0.015 M AgNO3 solution, there is a consumption of 105. Accordingly, calculate the percentage of Kl in the mixture A)88,4 B)43,7 C)56,3 D)11,6
- A 20.00 mL aliquot of lactic acid solution (HCH3H5O3) was titrated with 0.0980 M KOH(aq) using both an indicator and a pH meter. Ka (HCH3H5O3), is 1.38 x10-4. A total of 28.64 mL of 0.0980 M KOH(aq) was required to reach the equivalence point 1. Calculate the molarity of the lactic acid solution. 2. Calculate the pH of the lactic acid solution 3. Calculate the pH and [CH3H5O3-] at the half-equivalence point. 4. Calculate the pH at the equivalence point of the titration. 5. Suggest an appropriate indicator for titration. 6. Calculate the pH of the solution after 10.00 mL of 0.0980 M NaOH(aq) was addedWhat is the maximum volume (ml) of 0.195 M Ca(OH)2that can be titrated into 50.0 ml of a buffer comprising0.337 M acetic acid and 0.235 M sodium acetate,before depleting the buffer capacity.Give your answer to 3 significant figures?A 25.0-mL aliquot of vinegar was diluted to 250 mL in a volumetric flask. Titration of 50.0-mL aliquotsof the diluted solution required an average of 35.23 mL of 0.08960 M NaOH. Express the acidity of the vinegar in terms of the percentage (w/v) of acetic acid.
- What is the molarity of a commercial phosphoric acid solution, H3PO4 (MW= 98.0 g/mol) that is 85.0% (w/w) and has a specific gravity of 1.689? Also Make a graph of pH versus VHCl for the titrationYou are titrating 1.0 mL of a saturated calcium iodate Ca(IO3)2 solution in 0.0100 M potassium iodate KIO3 solution. At the endpoint, 0.78 mL of titrant (0.200 M Na2S2O3) was added. What is the total concentration of IO3-?Titration of 0.1615 g of an unknown monoprotic acid dissolved in 25.00 mL of water requires 21.84 mL of 0.1231 M NaOH to reach the endpoint. What is the molar mass of the acid?

