net free energy of coupled glucose breakdown and ATP synthesis be more or less favorable than the answer for net free energy of coupled glucose breakdown and ATP synthesis in organism in standard conditions. Please explain answer
net free energy of coupled glucose breakdown and ATP synthesis be more or less favorable than the answer for net free energy of coupled glucose breakdown and ATP synthesis in organism in standard conditions. Please explain answer
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 12P
Related questions
Question
Imagine that the concentrations of reactants and products for the coupled reactions above in the cell are at a level that yields a smaller molar ratio of the concentrations than those relevant to the standard state conditions. Would the net free energy of coupled glucose breakdown and ATP synthesis be more or less favorable than the answer for net free energy of coupled glucose breakdown and ATP synthesis in organism in standard conditions. Please explain answer
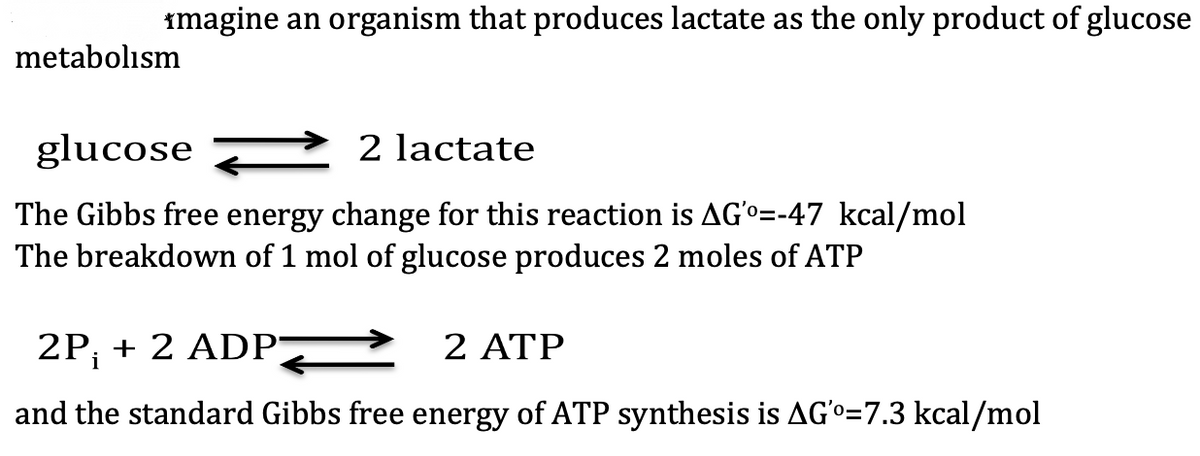
Transcribed Image Text:imagine an organism that produces lactate as the only product of glucose
metabolism
glucose
2 lactate
The Gibbs free energy change for this reaction is AG'o=-47 kcal/mol
The breakdown of 1 mol of glucose produces 2 moles of ATP
2P; + 2 ADP
2 ATP
and the standard Gibbs free energy of ATP synthesis is AGo=7.3 kcal/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning