In order to understand the concepts of pH and buffers, gain experience in using the Henderson-Hasselbalch equation, and to make the buffers you will be using this semester, you will make: 50.0 mL of 10X P, Buffer: 50.0 ml of 25X TE Buffer: 200 mM phosphate at pH 5.6 250 mM Tris with 25 mM EDTA at pH 7.5 The phosphate buffer will be prepared using a solid monobasic phosphate salt, and a solid dibasic phosphate salt. The same pK, works for either the Na* or K* salts. These salts are frequently hydrates, so the specific molar mass is provided.
In order to understand the concepts of pH and buffers, gain experience in using the Henderson-Hasselbalch equation, and to make the buffers you will be using this semester, you will make: 50.0 mL of 10X P, Buffer: 50.0 ml of 25X TE Buffer: 200 mM phosphate at pH 5.6 250 mM Tris with 25 mM EDTA at pH 7.5 The phosphate buffer will be prepared using a solid monobasic phosphate salt, and a solid dibasic phosphate salt. The same pK, works for either the Na* or K* salts. These salts are frequently hydrates, so the specific molar mass is provided.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.7QE
Related questions
Question
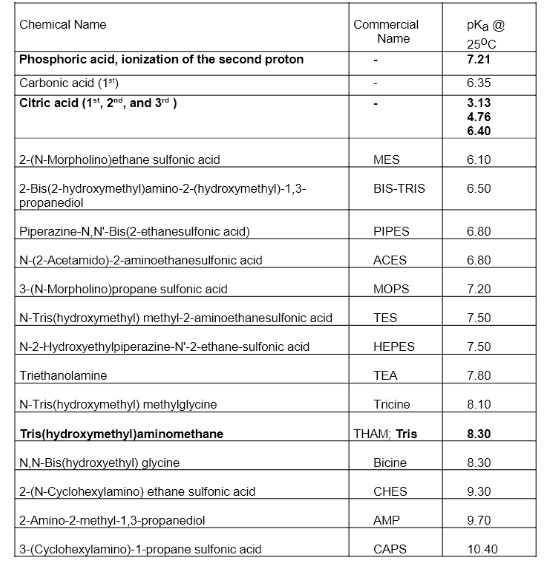
Calculate how to make a phosphate buffer. Calculate the concentrations of each species involved and then calculate the mass and/or volumes of the components required to prepare the buffer.
The Phosphate buffer will be in grams of each component.
Below is the necessary information. Please and thank you!
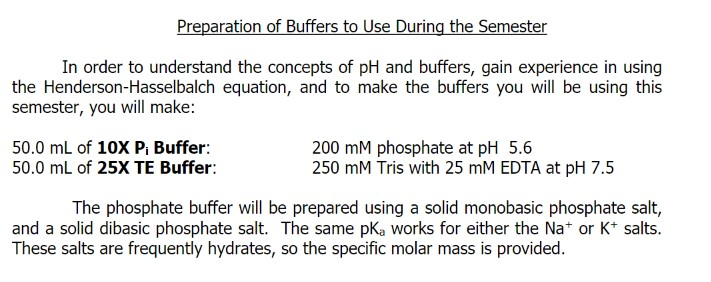
Transcribed Image Text:In order to understand the concepts of pH and buffers, gain experience in using
the Henderson-Hasselbalch equation, and to make the buffers you will be using this
semester, you will make:
50.0 mL of 10X P, Buffer:
50.0 ml of 25X TE Buffer:
200 mM phosphate at pH 5.6
250 mM Tris with 25 mM EDTA at pH 7.5
The phosphate buffer will be prepared using a solid monobasic phosphate salt,
and a solid dibasic phosphate salt. The same pK, works for either the Na* or K* salts.
These salts are frequently hydrates, so the specific molar mass is provided.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning