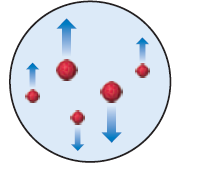
In the Millikan oil-drop experiment (see Figure 2.5), the tinyoil drops are observed through the viewing lens as rising,stationary, or falling, as shown here. (a) What causes theirrate of fall to vary from their rate in the absence of an electricfield? (b) Why do some drops move upward? [Section 2.2] The following exercises are divided into sections that dealwith specific topics in the chapter. The exercises are groupedin pairs, with the answers given in the back of the book tothe odd-numbered exercises, as indicated by the red exercisenumbers. Those exercises whose numbers appear in bracketsare more challenging than the nonbracketed exercises.
In the Millikan oil-drop experiment (see Figure 2.5), the tinyoil drops are observed through the viewing lens as rising,stationary, or falling, as shown here. (a) What causes theirrate of fall to vary from their rate in the absence of an electricfield? (b) Why do some drops move upward? [Section 2.2] The following exercises are divided into sections that dealwith specific topics in the chapter. The exercises are groupedin pairs, with the answers given in the back of the book tothe odd-numbered exercises, as indicated by the red exercisenumbers. Those exercises whose numbers appear in bracketsare more challenging than the nonbracketed exercises.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 38AP: In a reproduction of the Millikan oil-drop experiment, a student obtains the following values for...
Related questions
Question
In the Millikan oil-drop experiment (see Figure 2.5), the tiny
oil drops are observed through the viewing lens as rising,
stationary, or falling, as shown here. (a) What causes their
rate of fall to vary from their rate in the absence of an electric
field? (b) Why do some drops move upward? [Section 2.2]
The following exercises are divided into sections that deal
with specific topics in the chapter. The exercises are grouped
in pairs, with the answers given in the back of the book to
the odd-numbered exercises, as indicated by the red exercisenumbers. Those exercises whose numbers appear in brackets
are more challenging than the nonbracketed exercises.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning